Abstract
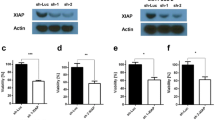
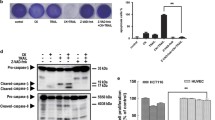
Mitochondria mediated signalling is the more common way of apoptosis induction exhibited by many chemotherapeutic agents in cancer cells. Death receptor mediated signalling for apoptosis in many cells also requires further amplification from the mitochondrial pathway activation through tBid. Thus the potential of most chemotherapeutic agents in tumours with intrinsic apoptosis resistance due to changes in molecules involved in the mitochondrial pathway is limited. Diaminothiazoles were shown earlier to bind to tubulin thereby exhibiting cytotoxicity towards different cancer cells. We observed that the lead diaminothiazole, DAT1 [4-amino-5-benzoyl-2-(4-methoxy phenyl amino) thiazole] could induce apoptosis in the colon cancer cell line HCT116 by both pathways. However, in contrast to many other chemotherapeutic agents, DAT1 triggered apoptosis where the intrinsic pathway was blocked by changing the pro and antiapoptotic proteins. An independent extrinsic pathway activation triggered by the upregulation of DR5 receptor accounted for that. The induction of DR5 occurred in the transcriptional level and the essential role of DR5 was confirmed by the fact that siRNA downregulation of DR5 significantly reduced DAT1 induced apoptosis. HCT116 cells were earlier shown to have a type II response for apoptosis induction where extrinsic pathway was connected to the intrinsic pathway via the mediator protein tBid. Our finding thus indicates that the signalling events in the manifestation of apoptosis depend not only on the cancer cell type, but also on the inducer. Our results also place diaminothiazoles in a promising position in the treatment of tumours with compromised apoptotic factors.









Similar content being viewed by others
References
Strasser A, O’Connor L, Dixit VM (2000) Apoptosis signaling. Annu Rev Biochem 69:217–245
Scaffidi C, Fulda S, Srinivasan A et al (1998) Two CD95 (APO-1/Fas) signaling pathways. EMBO J 17:1675–1687
Yin XM (2000) Signal transduction mediated by Bid, a pro-death Bcl-2 family proteins, connects the death receptor and mitochondria apoptosis pathways. Cell Res 10:161–167
Youle RJ, Strasser A (2008) The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9:47–59
Willis SN, Fletcher JI, Kaufmann T et al (2007) Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 315:856–859
Youle RJ (2007) Cell biology. Cellular demolition and the rules of engagement. Science 315:776–777
Mitchison TJ (1988) Microtubule dynamics and kinetochore function in mitosis. Annu Rev Cell Biol 4:527–549
Blagosklonny MV (2001) Unwinding the loop of Bcl-2 phosphorylation. Leukemia 15:869–874
Konishi Y, Lehtinen M, Donovan N, Bonni A (2002) Cdc2 phosphorylation of BAD links the cell cycle to the cell death machinery. Mol Cell 9:1005–1016
Yamaguchi H, Paranawithana SR, Lee MW, Huang Z, Bhalla KN, Wang HG (2002) Epothilone B analogue (BMS-247550)-mediated cytotoxicity through induction of Bax conformational change in human breast cancer cells. Cancer Res 62:466–471
Ruvolo PP, Deng X, May WS (2001) Phosphorylation of Bcl2 and regulation of apoptosis. Leukemia 15:515–522
Yamaguchi H, Chen J, Bhalla K, Wang HG (2004) Regulation of Bax activation and apoptotic response to microtubule-damaging agents by p53 transcription-dependent and -independent pathways. J Biol Chem 279:39431–39437
Chandrika BB, Maney SK, Lekshmi SU et al (2010) Bax deficiency mediated drug resistance can be reversed by endoplasmic reticulum stress induced death signaling. Biochem Pharmacol 79:1589–1599
Gobe G, Rubin M, Williams G, Sawczuk I, Buttyan R (2002) Apoptosis and expression of Bcl-2, Bcl-XL, and Bax in renal cell carcinomas. Cancer Invest 20:324–332
Yagi OK, Akiyama Y, Nomizu T, Iwama T, Endo M, Yuasa Y (1998) Proapoptotic gene BAX is frequently mutated in hereditary nonpolyposis colorectal cancers but not in adenomas. Gastroenterology 114:268–274
Gazitt Y, Rothenberg ML, Hilsenbeck SG, Fey V, Thomas C, Montegomrey W (1998) Bcl-2 overexpression is associated with resistance to paclitaxel, but not gemcitabine, in multiple myeloma cells. Int J Oncol 13:839–848
Upreti M, Chu R, Galitovskaya E, Smart SK, Chambers TC (2008) Key role for Bak activation and Bak-Bax interaction in the apoptotic response to vinblastine. Mol Cancer Ther 7:2224–2232
Chu SS, Alegria LA, Bender SL et al (2003) Thiazole compounds and pharmaceutical compositions for inhibiting protein kinases and methods for their use. US Patent 6(620):828
Romagnoli R, Baraldi PG, Carrion MD et al (2009) 2-Arylamino-4-amino-5-aroylthiazoles. “One-pot” synthesis and biological evaluation of a new class of inhibitors of tubulin polymerization. J Med Chem 52:5551–5555
Sengupta S, Smitha SL, Thomas NE et al (2005) 4-Amino-5-benzoyl-2-(4-methoxyphenylamino)thiazole (DAT1): a cytotoxic agent towards cancer cells and a probe for tubulin-microtubule system. Br J Pharmacol 145:1076–1083
Thomas SA, Thamkachy R, Ashokan B et al (2012) Diaminothiazoles inhibit angiogenesis efficiently by suppressing Akt phosphorylation. J Pharmacol Exp Ther 341:718–724
Sreejalekshmi KG, Devi SKC, Rajasekharan KN (2006) An efficient protocol for solid phase aminothiazole synthesis. Tetrahedron Lett 47:6179–6182
Gajate C, Barasoain I, Andreu JM, Mollinedo F (2000) Induction of apoptosis in leukemic cells by the reversible microtubule-disrupting agent 2-methoxy-5-(2′,3′,4′-trimethoxyphenyl)-2,4,6-cycloheptatrien-1 -one: protection by Bcl-2 and Bcl-X(L) and cell cycle arrest. Cancer Res 60:2651–2659
Jordan MA (2002) Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr Med Chem Anticancer Agents 2:1–17
Liu X, Kim CN, Yang J, Jemmerson R, Wang X (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86:147–157
Li P, Nijhawan D, Budihardjo I et al (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479–489
Breitschopf K, Haendeler J, Malchow P, Zeiher AM, Dimmeler S (2000) Posttranslational modification of Bcl-2 facilitates its proteasome-dependent degradation: molecular characterization of the involved signaling pathway. Mol Cell Biol 20:1886–1896
Haldar S, Jena N, Croce CM (1995) Inactivation of Bcl-2 by phosphorylation. Proc Natl Acad Sci U S A 92:4507–4511
Blagosklonny MV, Schulte T, Nguyen P, Trepel J, Neckers LM (1996) Taxol-induced apoptosis and phosphorylation of Bcl-2 protein involves c-Raf-1 and represents a novel c-Raf-1 signal transduction pathway. Cancer Res 56:1851–1854
Scatena CD, Stewart ZA, Mays D et al (1998) Mitotic phosphorylation of Bcl-2 during normal cell cycle progression and Taxol-induced growth arrest. J Biol Chem 273:30777–30784
Ray CA, Black RA, Kronheim SR et al (1992) Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell 69:597–604
Tewari M, Telford WG, Miller RA, Dixit VM (1995) CrmA, a poxvirus-encoded serpin, inhibits cytotoxic T-lymphocyte-mediated apoptosis. J Biol Chem 270:22705–22708
Jordan MA, Wilson L (2004) Microtubules as a target for anticancer drugs. Nat Rev Cancer 4:253–265
Zhou J, Giannakakou P (2005) Targeting microtubules for cancer chemotherapy. Curr Med Chem Anticancer Agents 5:65–71
Blagosklonny V (2007) Mitotic arrest and cell fate: why and how mitotic inhibition of transcription drives mutually exclusive events. Cell Cycle 6:70–74
Nimmanapalli R, Perkins CL, Orlando M, O’Bryan E, Nguyen D, Bhalla KN (2001) Pretreatment with paclitaxel enhances apo-2 ligand/tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis of prostate cancer cells by inducing death receptors 4 and 5 protein levels. Cancer Res 61:759–763
von Haefen C, Wieder T, Essmann F, Schulze-Osthoff K, Dorken B, Daniel PT (2003) Paclitaxel-induced apoptosis in BJAB cells proceeds via a death receptor-independent, caspases-3/-8-driven mitochondrial amplification loop. Oncogene 22:2236–2247
Mooberry SL (2003) New insights into 2-methoxyestradiol, a promising antiangiogenic and antitumor agent. Curr Opin Oncol 15:425–430
LaVallee TM, Zhan XH, Johnson MS et al (2003) 2-methoxyestradiol up-regulates death receptor 5 and induces apoptosis through activation of the extrinsic pathway. Cancer Res 63:468–475
Chang YH, Yang JS, Kuo SC, Chung JG (2009) Induction of mitotic arrest and apoptosis by a novel synthetic quinolone analogue, CWC-8, via intrinsic and extrinsic apoptotic pathways in human osteogenic sarcoma U-2 OS cells. Anticancer Res 29:3139–3148
Krajewska M, Fenoglio-Preiser CM, Krajewski S et al (1996) Immunohistochemical analysis of Bcl-2 family proteins in adenocarcinomas of the stomach. Am J Pathol 149:1449–1457
Rampino N, Yamamoto H, Ionov Y et al (1997) Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science 275:967–969
Yamaguchi H, Bhalla K, Wang HG (2003) Bax plays a pivotal role in thapsigargin-induced apoptosis of human colon cancer HCT116 cells by controlling Smac/Diablo and Omi/HtrA2 release from mitochondria. Cancer Res 63:1483–1489
Igney FH, Krammer PH (2002) Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer 2:277–288
Lee JM, Bernstein A (1995) Apoptosis, cancer and the p53 tumour suppressor gene. Cancer Metastasis Rev 14:149–161
Ashkenazi A (2008) Targeting the extrinsic apoptosis pathway in cancer. Cytokine Growth Factor Rev 19:325–331
Acknowledgments
The authors acknowledge Dr Julian Downward (London Research Institute) for the Bcl2 EGFP and BclXL EGFP plasmids, Dr Vishwa M. Dixit (University of California, SF) for the pcDNA3 CrmA plasmid and Dr Bert Vogelstein (Johns Hopkins School of Medicine) for the Bax deficient derivative of HCT116. The authors are also thankful for the financial support of R.G.C.B. core funding from Department of Biotechnology, Govt. of India and to the Council of Scientific and Industrial Research, Department of Biotechnology and University Grants Commission, Govt. of India for fellowships to Thomas, Vasudevan and Thamkachy respectively.
Conflict of interest
The authors confirm that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sannu A. Thomas, Smreti Vasudevan and Reshma Thamkachy contributed equally in the work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Thomas, S.A., Vasudevan, S., Thamkachy, R. et al. Upregulation of DR5 receptor by the diaminothiazole DAT1 [4-amino-5-benzoyl-2-(4-methoxy phenyl amino) thiazole] triggers an independent extrinsic pathway of apoptosis in colon cancer cells with compromised pro and antiapoptotic proteins. Apoptosis 18, 713–726 (2013). https://doi.org/10.1007/s10495-013-0826-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-013-0826-6