Abstract
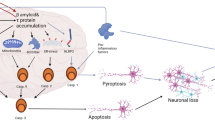
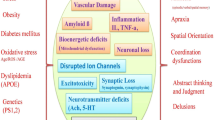
Although apoptosis plays a critical role in molding the CNS into its final appearance and function, inappropriate activation of this pathway in the aging brain may contribute to neurodegeneration. In Alzheimer’s disease (AD), an overwhelming body of evidence supports the activation of apoptosis in general, and caspases specifically as an early event that may not only contribute to neurodegeneration but also promote the underlying pathology associated with this disease. Therefore, caspase inhibitors may provide an effective strategy for treating AD. However, despite the compelling evidence indicating a role for caspases in disease progression, chronic treatment with caspase inhibitors in animal models of AD has never been undertaken. In this review the role of caspases in AD will be addressed, including recent studies utilizing in vivo transgenic mouse models of tauopathies. In addition, a discussion of the therapeutic value and dangers of targeting caspase inhibition in the treatment of AD using caspase inhibitors such as Q-VD-OPh will be evaluated.


Similar content being viewed by others
References
Alzheimer’s Association (2009) Alzheimer’s disease facts and figures. Alzheimers Dement 5(3):234–270
Duyckaerts C, Delatour B, Potier MC (2009) Classification and basic pathology of Alzheimer disease. Acta Neuropathol 118:5–36
Mirra SS, Heyman A, McKeel D et al (1991) The consortium to establish a registry for Alzheimer’s disease (CERAD) part II. Standardization of the neuropathological assessment of Alzheimer’s disease. Neurol 41:479–486
Trojanowski JQ, Schmidt ML, Shin R-W, Bramblett GT, Goedert M, Lee M-Y (1993) From pathological marker to potential mediator of neuronal dysfunction and degeneration in Alzheimer’s disease. Clin Neurosci 1:184–191
Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VM (1993) Abnormal tau phosphorylation at Ser396 in Alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron 10:1089–1099
Golde TE, Dickson D, Hutton M (2006) Filling the gaps in the abeta cascade hypothesis of Alzheimer’s disease. Curr Alzheimer Res 3:421–430
Lambert MP, Barlow AK, Chromy BA et al (1998) Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA 95:6448–6453
Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT (1992) Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 42:631–639
Price JL, Morris JC (1999) Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol 45:358–368
Su JH, Anderson AJ, Cummings BJ, Cotman CW (1994) Immunohistochemical evidence for DNA fragmentation in neurons in the AD brain. Neuroreport 5:2529–2533
Charriaut-Marlangue C, Ben-Ari Y (1995) A cautionary note on the use of the TUNEL stain to determine apoptosis. Neuroreport 7:61–64
Yang F, Sun X, Beech W et al (1998) Antibody to caspase-cleaved actin detects apoptosis in differentiated neuroblastoma and plaque-associated neurons and microglia in Alzheimer’s disease (see comments). Am J Pathol 152:379–389
Gervais FG, Xu D, Robertson GS et al (1999) Involvement of caspases in proteolytic cleavage of Alzheimer’s amyloid- beta precursor protein and amyloidogenic A beta peptide formation. Cell 97:395–406
Goodman SR, Zagon IS, Coleman DB, McLaughlin PJ (1986) Spectrin expression in neuroblastoma cells. Brain Res Bull 16:597–602
Rohn TT, Head E, Su JH et al (2001) Correlation between caspase activation and neurofibrillary tangle formation in Alzheimer’s disease. Am J Pathol 158:189–198
Canu N, Dus L, Barbato C et al (1998) Tau cleavage and dephosphorylation in cerebellar granule neurons undergoing apoptosis. J Neurosci 18:7061–7074
Rohn TT, Rissman RA, Davis MC, Kim Y-E, Cotman C, Head E (2002) Caspase-9 activation and caspase cleavage of tau in the Alzheimer’s disease brain. Neurobiol Dis 11:341–354
Rohn TT, Rissman RA, Head E, Cotman CW (2002) Caspase activation in the Alzheimer’s disease brain: tortuous and torturous. Drug News Perspect 15:549–557
Gamblin TC, Chen F, Zambrano A et al (2003) Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci USA 100:10032–10037
Rissman RA, Poon WW, Blurton-Jones M et al (2004) Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest 114:121–130
Guo H, Albrecht S, Bourdeau M, Petzke T, Bergeron C, LeBlanc AC (2004) Active caspase-6 and caspase-6-cleaved tau in neuropil threads, neuritic plaques, and neurofibrillary tangles of Alzheimer’s disease. Am J Pathol 165:523–531
Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM (2003) Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol Aging 24:1063–1070
Oddo S, Caccamo A, Shepherd JD et al (2003) Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 39:409–421
Rohn TT, Vyas V, Hernandez-Estrada T, Nichol KE, Christie LA, Head E (2008) Lack of pathology in a triple transgenic mouse model of Alzheimer’s disease after overexpression of the anti-apoptotic protein Bcl-2. J Neurosci 28:3051–3059
Spires-Jones TL, de Calignon A, Matsui T et al (2008) In vivo imaging reveals dissociation between caspase activation and acute neuronal death in tangle-bearing neurons. J Neurosci 28:862–867
de Calignon A, Spires-Jones TL, Pitstick R, Carlson GA, Hyman BT (2009) Tangle-bearing neurons survive despite disruption of membrane integrity in a mouse model of tauopathy. J Neuropathol Exp Neurol 68:757–761
Li M, Ona VO, Guegan C et al (2000) Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model (see comments). Science 288:335–339
Ona VO, Li M, Vonsattel JP et al (1999) Inhibition of caspase-1 slows disease progression in a mouse model of Huntington’s disease. Nature 399:263–267
Schierle GS, Hansson O, Leist M, Nicotera P, Widner H, Brundin P (1999) Caspase inhibition reduces apoptosis and increases survival of nigral transplants. Nat Med 5:97–100
Friedlander RM (2003) Apoptosis and caspases in neurodegenerative diseases. N Engl J Med 348:1365–1375
Van Noorden CJ (2001) The history of Z-VAD-FMK, a tool for understanding the significance of caspase inhibition. Acta Histochem 103:241–251
Caserta TM, Smith AN, Gultice AD, Reedy MA, Brown TL (2003) Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis 8:345–352
Chauvier D, Ankri S, Charriaut-Marlangue C, Casimir R, Jacotot E (2007) Broad-spectrum caspase inhibitors: from myth to reality? Cell Death Differ 14:387–391
Yang L, Sugama S, Mischak RP et al (2004) A novel systemically active caspase inhibitor attenuates the toxicities of MPTP, malonate, and 3NP in vivo. Neurobiol Dis 17:250–259
Braun JS, Prass K, Dirnagl U, Meisel A, Meisel C (2007) Protection from brain damage and bacterial infection in murine stroke by the novel caspase-inhibitor Q-VD-OPH. Exp Neurol 206:183–191
Kim HS, Suh YH (2009) Minocycline and neurodegenerative diseases. Behav Brain Res 196:168–179
Gordon PH, Moore DH, Miller RG et al (2007) Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol 6:1045–1053
Choi Y, Kim HS, Shin KY et al (2007) Minocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer’s disease models. Neuropsychopharmacology 32(11):2393–2404
Fan R, Xu F, Previti ML et al (2007) Minocycline reduces microglial activation and improves behavioral deficits in a transgenic model of cerebral microvascular amyloid. J Neurosci 27:3057–3063
Seabrook TJ, Jiang L, Maier M, Lemere CA (2006) Minocycline affects microglia activation, Abeta deposition, and behavior in APP-tg mice. Glia 53:776–782
Noble W, Garwood C, Stephenson J, Kinsey AM, Hanger DP, Anderton BH (2009) Minocycline reduces the development of abnormal tau species in models of Alzheimer’s disease. Faseb J 23:739–750
Zhu S, Stavrovskaya IG, Drozda M et al (2002) Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature 417:74–78
Teng YD, Choi H, Onario RC et al (2004) Minocycline inhibits contusion-triggered mitochondrial cytochrome c release and mitigates functional deficits after spinal cord injury. Proc Natl Acad Sci USA 101:3071–3076
Tikka TM, Koistinaho JE (2001) Minocycline provides neuroprotection against N-methyl-D-aspartate neurotoxicity by inhibiting microglia. J Immunol 166:7527–7533
Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J (2001) Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci 21:2580–2588
Streit WJ (2005) Microglia and neuroprotection: implications for Alzheimer’s disease. Brain Res Brain Res Rev 48:234–239
Pockros PJ, Schiff ER, Shiffman ML et al (2007) Oral IDN-6556, an antiapoptotic caspase inhibitor, may lower aminotransferase activity in patients with chronic hepatitis C. Hepatology 46:324–329
Investigators NN-P (2008) A pilot clinical trial of creatine and minocycline in early Parkinson disease: 18-month results. Clin Neuropharmacol 31:141–150
Frisoni GB, Delacourte A (2009) Neuroimaging outcomes in clinical trials in Alzheimer’s disease. J Nutr Health Aging 13:209–212
Butterfield DA (2002) Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Radic Res 36:1307–1313
Smith DG, Cappai R, Barnham KJ (2007) The redox chemistry of the Alzheimer’s disease amyloid beta peptide. Biochim Biophys Acta 1768:1976–1990
Acknowledgements
Funded by a grant from the American Health Assistance Foundation [AHAF] and a grant from the KO Alzheimer’s Dementia Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rohn, T.T. The role of caspases in Alzheimer’s disease; potential novel therapeutic opportunities. Apoptosis 15, 1403–1409 (2010). https://doi.org/10.1007/s10495-010-0463-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-010-0463-2