Abstract
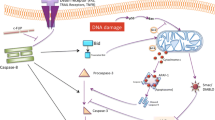
Death receptor-dependent apoptosis is an important mechanism of growth control. It has been demonstrated that Ras association domain family protein 1A (RASSF1A) is a tumor suppressor protein involved in death receptor-dependent apoptosis. However, it is unclear how RASSF1A-mediated cell death is initiated. We have now detailed 14-3-3 dependent regulation of RASSF1A-mediated cell death. We demonstrate that basal association of RASSF1A with 14-3-3 was lost following stimulation with tumor necrosis factor alpha (TNFα) or TNFα related apoptosis inducing ligand (TRAIL). Subsequent to the loss of 14-3-3 association, RASSF1A associated with modulator of apoptosis (MOAP-1) followed by death receptor association with either TNFα receptor 1 (TNF-R1) or TRAIL receptor 1 (TRAIL-R1). 14-3-3 association required basal phosphorylation by the serine/threonine kinase, glycogen synthase kinase 3β (GSK-3β), on serine 175, 178, and 179. Mutation of these critical serines resulted in the loss of 14-3-3 association and earlier recruitment of RASSF1A to MOAP-1, TNF-R1, and TRAIL-R1. Furthermore, stable cells containing a triple serine mutant of RASSF1A [serine (S) 175 to alanine (A) [S175A], S178A, and S179A] resulted in increased basal cell death, enhanced Annexin V staining and enhanced cleavage of poly (ADP-ribose) polymerase (PARP) following TNFα stimulation when compared to stable cells containing wild type RASSF1A. RASSF1A-mediated cell death is, therefore, tightly controlled by 14-3-3 association.






Similar content being viewed by others
References
Aitken A (2006) 14-3-3 Proteins: a historic overview. Semin Cancer Biol 16:162–172
Berg D, Holzmann C, Riess O (2003) 14-3-3 proteins in the nervous system. Nat Rev Neurosci 4:752–762
Rosenquist M (2003) 14-3-3 proteins in apoptosis. Braz J Med Biol Res 36:403–408
Tzivion G, Shen YH, Zhu J (2001) 14-3-3 proteins; bringing new definitions to scaffolding. Oncogene 20:6331–6338
Wilker EW, van Vugt MA, Artim SA et al (2007) 14-3-3 sigma controls mitotic translation to facilitate cytokinesis. Nature 446:329–332
Gardino AK, Smerdon SJ, Yaffe MB (2006) Structural determinants of 14-3-3 binding specificities and regulation of subcellular localization of 14-3-3-ligand complexes: a comparison of the X-ray crystal structures of all human 14-3-3 isoforms. Semin Cancer Biol 16:173–182
Borch J, Bych K, Roepstorff P, Palmgren MG, Fuglsang AT (2002) Phosphorylation-independent interaction between 14-3-3 protein and the plant plasma membrane H+-ATPase. Biochem Soc Trans 30:411–415
Aitken A, Baxter H, Dubois T et al (2002) Specificity of 14-3-3 isoform dimer interactions and phosphorylation. Biochem Soc Trans 30:351–360
Wilker EW, Grant RA, Artim SC, Yaffe MB (2005) A structural basis for 14-3-3 sigma functional specificity. J Biol Chem 280:18891–18898
Lal G, Padmanabha L, Provenzano M, Fitzgerald M, Weydert J, Domann FE (2008) Regulation of 14-3-3 sigma expression in human thyroid carcinoma is epigenetically regulated by aberrant cytosine methylation. Cancer Lett 267:165–174
Suzuki H, Itoh F, Toyota M, Kikuchi T, Kakiuchi H, Imai K (2000) Inactivation of the 14-3-3 sigma gene is associated with 5′ CpG island hypermethylation in human cancers. Cancer Res 60:4353–4357
Richter AM, Pfeifer GP, Dammann RH (2009) The RASSF proteins in cancer; from epigenetic silencing to functional characterization. Biochim Biophys Acta 1796:114–128
Avruch J, Xavier R, Bardeesy N et al (2009) Rassf family of tumor suppressor polypeptides. J Biol Chem 284:11001–11005
Dammann R, Schagdarsurengin U, Seidel C et al (2005) The tumor suppressor RASSF1A in human carcinogenesis: an update. Histol Histopathol 20:645–663
van der Weyden L, Adams DJ (2007) The Ras-association domain family (RASSF) members and their role in human tumourigenesis. Biochim Biophys Acta 1776:58–85
Allen NP, Donninger H, Vos MD et al (2007) RASSF6 is a novel member of the RASSF family of tumor suppressors. Oncogene 26:6203–6211
Song MS, Chang JS, Song SJ, Yang TH, Lee H, Lim DS (2005) The centrosomal protein RAS association domain family protein 1A (RASSF1A)-binding protein 1 regulates mitotic progression by recruiting RASSF1A to spindle poles. J Biol Chem 280:3920–3927
Song MS, Song SJ, Ayad NG et al (2004) The tumour suppressor RASSF1A regulates mitosis by inhibiting the APC-Cdc20 complex. Nat Cell Biol 6:129–137
Estrabaud E, Lassot I, Blot G et al (2007) RASSF1C, an isoform of the tumor suppressor RASSF1A, promotes the accumulation of {beta}-catenin by interacting with {beta}TrCP. Cancer Res 67:1054–1061
Vos MD, Ellis CA, Elam C, Ulku AS, Taylor BJ, Clark GJ (2003) RASSF2 is a novel K-Ras-specific effector and potential tumor suppressor. J Biol Chem 278:28045–28051
Matallanas D, Romano D, Yee K et al (2007) RASSF1A elicits apoptosis through an MST2 pathway directing proapoptotic transcription by the p73 tumor suppressor protein. Mol Cell 27:962–975
Oh HJ, Lee KK, Song SJ et al (2006) Role of the tumor suppressor RASSF1A in Mst1-mediated apoptosis. Cancer Res 66:2562–2569
Praskova M, Khoklatchev A, Ortiz-Vega S, Avruch J (2004) Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem J 381:453–462
Baksh S, Tommasi S, Fenton S et al (2005) The tumor suppressor RASSF1A and MAP-1 link death receptor signaling to Bax conformational change and cell death. Mol Cell 18:637–650
Foley CJ, Freedman H, Choo SL et al (2008) Dynamics of RASSF1A/MOAP-1 association with death receptors. Mol Cell Biol 28:4520–4535
Tan KO, Fu NY, Sukumaran SK et al (2005) MAP-1 is a mitochondrial effector of Bax. Proc Natl Acad Sci U S A 102:14623–14628
Verma SK, Ganesan TS, Parker PJ (2008) The tumour suppressor RASSF1A is a novel substrate of PKC. FEBS Lett 582:2270–2276
Feyt C, Kienlen-Campard P, Leroy K et al (2005) Lithium chloride increases the production of amyloid-beta peptide independently from its inhibition of glycogen synthase kinase 3. J Biol Chem 280:33220–33227
Korur S, Huber RM, Sivasankaran B et al (2009) GSK3beta regulates differentiation and growth arrest in glioblastoma. PloS one 4:e7443
Al-Assar O, Crouch DH (2005) Inactivation of MAP kinase signalling in Myc transformed cells and rescue by LiCl inhibition of GSK3. Molecular cancer 4:13
Laurenz JC, Smith SB (1998) Lithium chloride does not inhibit the proliferation of L6 myoblasts by decreasing intracellular free inositol. J Anim Sci 76:66–73
Tritsaris K, Gromada J, Jorgensen TD, Nauntofte B, Dissing S (2001) Reduction in the rate of inositol 1,4,5-trisphosphate synthesis in rat parotid acini by lithium. Arch Oral Biol 46:365–373
Dougherty MK, Morrison DK (2004) Unlocking the code of 14-3-3. J Cell Sci 117:1875–1884
Adachi M, Zhang YB, Imai K (2003) Mutation of BAD within the BH3 domain impairs its phosphorylation-mediated regulation. FEBS Lett 551:147–152
Nomura M, Shimizu S, Sugiyama T et al (2003) 14-3-3 Interacts directly with and negatively regulates pro-apoptotic Bax. J Biol Chem 278:2058–2065
Samuel T, Weber HO, Rauch P et al (2001) The G2/M regulator 14-3-3sigma prevents apoptosis through sequestration of Bax. J Biol Chem 276:45201–45206
Liu Y, Yin G, Surapisitchat J, Berk BC, Min W (2001) Laminar flow inhibits TNF-induced ASK1 activation by preventing dissociation of ASK1 from its inhibitor 14-3-3. J Clin Invest 107:917–923
Zhang H, Zhang H, Lin Y, Li J, Pober JS, Min W (2007) RIP1-mediated AIP1 phosphorylation at a 14-3-3-binding site is critical for tumor necrosis factor-induced ASK1-JNK/p38 activation. J Biol Chem 282:14788–14796
Klein PS, Melton DA (1996) A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A 93:8455–8459
Chin PC, Majdzadeh N, D’Mello SR (2005) Inhibition of GSK3beta is a common event in neuroprotection by different survival factors. Brain Res Mol Brain Res 137:193–201
Whitehurst AW, Ram R, Shivakumar L, Gao B, Minna JD, White MA (2008) The RASSF1A tumor suppressor restrains anaphase-promoting complex/cyclosome activity during the G1/S phase transition to promote cell cycle progression in human epithelial cells. Mol Cell Biol 28:3190–3197
Song MS, Song SJ, Kim SJ, Nakayama K, Nakayama KI, Lim DS (2008) Skp2 regulates the antiproliferative function of the tumor suppressor RASSF1A via ubiquitin-mediated degradation at the G1-S transition. Oncogene 27:3176–3185
Rong R, Jiang LY, Sheikh MS, Huang Y (2007) Mitotic kinase Aurora-A phosphorylates RASSF1A and modulates RASSF1A-mediated microtubule interaction and M-phase cell cycle regulation. Oncogene 26:7700–7708
Liu L, Guo C, Dammann R, Tommasi S, Pfeifer GP (2008) RASSF1A interacts with and activates the mitotic kinase Aurora-A. Oncogene 27:6175–6186
Sakanaka C (2002) Phosphorylation and regulation of beta-catenin by casein kinase I epsilon. J Biochem 132:697–703
Acknowledgments
We would like to thank Christina Onyskiw for her excellent technical assistance with our experiments. We would like to thank Dr. Victor Yu and NaiYang Fu for the MOAP-1 expression construct; Dr. Gerd Pfeifer for the GST expression constructs for RASSF1A, 1C, and RASSF5; Dr. Yutaka Hata for human Myc-tagged RASSF6B; Dr. Andrew Chalmers for human HA-tagged RASSF7; and Dr. Michael Yaffe for the HA-14-3-3 expression constructs. Grant support for this study was provided by the University of Alberta, Faculty of Medicine and Department of Pediatrics (N031000425) (SB); by the Women and Children’s Health Research Institute (WCO14) (SB, HA-G, JDO), Canadian Institutes of Health Research (MOP-79494) (CO), Alberta Heritage Foundation for Medical Research (G220170170) (SB), and Canadian Foundation for Innovation/Alberta Small Equipment Grants Program (13118); and by the Office of the Provost and VP Academic Award (SLC, RCC, DP and RXW).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10495_2009_451_MOESM2_ESM.pdf
Supplemental Fig. S1 Specificity of RASSF1A/14-3-3 associations. a and b, HA (a) or GST (b) tagged RASSF proteins were ectopically expressed in U2OS cells. Associated RASSF isoforms were recovered by 14-3-3 immunoprecipitation (IP) followed by immunoblotting (IB) with the indicated antibodies. (PDF 119 kb)
10495_2009_451_MOESM3_ESM.pdf
Supplemental Fig. S2 Loss of RASSF1A self association with 14-3-3 binding site mutants. Green fluorescent protein (GFP) tagged RASSF1A and HA tagged RASSF1A WT or serine mutants were ectopically expressed in U2OS cells. Associated GFP-RASSF1A was recovered by immunoprecipitation with an anti-HA antibody followed by immunoblotting with the indicated antibodies. (PDF 14 kb)
Rights and permissions
About this article
Cite this article
Ghazaleh, H.A., Chow, R.S., Choo, S.L. et al. 14-3-3 Mediated regulation of the tumor suppressor protein, RASSF1A. Apoptosis 15, 117–127 (2010). https://doi.org/10.1007/s10495-009-0451-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-009-0451-6