Abstract
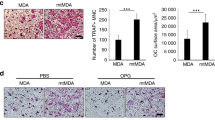
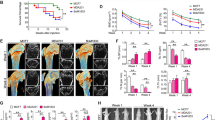
We compared the effect of conditioned medium (CM) from several human breast carcinoma cell lines on osteoclast bone resorbing activity and osteoclast apoptosis. Our findings indicate that ability of cancer cell line to increase the in vitro bone resorbing activity is linked to their potential to inhibit osteoclast apoptosis. Cancer cells producing the higher level of M-CSF have the higher osteolytic activity, suggesting that M-CSF originating from cancer cells may contribute, at least in part, to the osteoclast activity at the metastatic site by enhancing their survival. Given that M-CSF plays an important role in the anti-apoptotic effect, we speculated that blocking M-CSF pathway would prevent the CM effects. Small interfering RNA (siRNA) targeting M-CSF and imatinib, a protein tyrosine kinase inhibitor targeting M-CSF receptor, almost completely reversed the CM effect on both osteoclast apoptosis and bone resorption. Blockade of M-CSF pathway could be thus of clinical value in the treatment of breast cancer related bone destruction.
Similar content being viewed by others
Abbreviations
- M-CSF:
-
macrophage-colony stimulating factor
- CM:
-
conditioned media
References
Mundy GR (1997) Mechanisms of bone metastasis. Cancer 80:1546–1556
Clohisy DR, Ogilvie CM, Carpenter RJ, Ramnaraine ML (1996) Localized, tumor-associated osteolysis involves the recruitment and activation of osteoclasts. J Orthop Res 14:2–6
Taube T, Elomaa I, Blomqvist C, Beneton MN, Kanis JA (1994) Histomorphometric evidence for osteoclast-mediated bone resorption in metastatic breast cancer. Bone 15:161–166
Guise TA, Mundy GR (1998) Cancer and bone. Endocr Rev 19:18–54
Mundy GR (2002) Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2:584–593
Yoneda T, Hiraga T (2005) Crosstalk between cancer cells and bone microenvironment in bone metastasis. Biochem Biophys Res Commun 328:679–687
Guise TA, Kozlow WM, Heras-Herzig A, Padalecki SS, Yin JJ, Chirgwin JM (2005) Molecular mechanisms of breast cancer metastases to bone. Clin Breast Cancer (5 Suppl):S46–53
Guise TA, Yin JJ, Taylor SD, et al (1996) Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest 98:1544–1549
Guise TA (1997) Parathyroid hormone-related protein and bone metastases. Cancer 80:1572–1580
Mancino AT, Klimberg VS, Yamamoto M, Manolagas SC, Abe E (2001) Breast cancer increases osteoclastogenesis by secreting M-CSF and upregulating RANKL in stromal cells. J Surg Res 100:18–24
De Larco JE, Wuertz BR, Rosner KA, et al (2001) A potential role for interleukin-8 in the metastatic phenotype of breast carcinoma cells. Am J Pathol 158:639–646
Bendre MS, Montague DC, Peery T, Akel NS, Gaddy D, Suva LJ (2003) Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone 33:28–37
Morinaga Y, Fujita N, Ohishi K, Tsuruo T (1997) Stimulation of interleukin-11 production from osteoblast-like cells by transforming growth factor-beta and tumor cell factors. Int J Cancer 71:422–428
Parfitt AM, Mundy GR, Roodman GD, Hughes DE, Boyce BF (1996) A new model for the regulation of bone resorption, with particular reference to the effects of bisphosphonates. J Bone Miner Res 11:150–159
Greenfield EM, Bi Y, Miyauchi A (1999) Regulation of osteoclast activity. Life Sci 65:1087–1102
Gallet M, Sevenet N, Dupont C, Brazier M, Kamel S (2004) Breast cancer cell line MDA-MB 231 exerts a potent and direct anti-apoptotic effect on mature osteoclasts. Biochem Biophys Res Commun 319:690–696
Tezuka K, Sato T, Kamioka H, et al (1992) Identification of osteopontin in isolated rabbit osteoclasts. Biochem Biophys Res Commun 186:911–917
Kameda T, Ishikawa H, Tsutsui T (1995) Detection and characterization of apoptosis in osteoclasts in vitro. Biochem Biophys Res Commun 207:753–760
Dewar AL, Cambareri AC, Zannettino AC, et al (2005) Macrophage colony-stimulating factor receptor c-fms is a novel target of imatinib. Blood 105:3127–3132
Manolagas SC (2000) Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21:115–137
Roodman GD (1999) Cell biology of the osteoclast. Exp Hematol 27:1229–1241
Clohisy DR, Palkert D, Ramnaraine ML, Pekurovsky I, Oursler MJ (1996) Human breast cancer induces osteoclast activation and increases the number of osteoclasts at sites of tumor osteolysis. J Orthop Res 14:396–402
Thomas RJ, Guise TA, Yin JJ, et al (1999) Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology 140:4451–4458
Kitazawa S, Kitazawa R (2002) RANK ligand is a prerequisite for cancer-associated osteolytic lesions. J Pathol 198:228–236
Fuller K, Owens JM, Jagger CJ, Wilson A, Moss R, Chambers TJ (1993) Macrophage colony-stimulating factor stimulates survival and chemotactic behavior in isolated osteoclasts. J Exp Med 178:1733–1744
Woo KM, Kim HM, Ko JS (2002) Macrophage colony-stimulating factor promotes the survival of osteoclast precursors by up-regulating Bcl-X(L). Exp Mol Med 34:340–346
Glantschnig H, Fisher JE, Wesolowski G, Rodan GA, Reszka AA (2003) M-CSF, TNFalpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ 10:1165–1177
Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW Jr, et al (1990) Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci USA 87:4828–4832
Reszka AA, Halasy-Nagy JM, Masarachia PJ, Rodan GA (1999) Bisphosphonates act directly on the osteoclast to induce caspase cleavage of mst1 kinase during apoptosis. A link between inhibition of the mevalonate pathway and regulation of an apoptosis-promoting kinase. J Biol Chem 274:34967–34973
van der Pluijm G, Sijmons B, Vloedgraven H, et al (2001) Urokinase-receptor/integrin complexes are functionally involved in adhesion and progression of human breast cancer in vivo. Am J Pathol 159:971–982
Carroll M, Ohno-Jones S, Tamura S, et al (1997) CGP 57148, a tyrosine kinase inhibitor, inhibits the growth of cells expressing BCR-ABL, TEL-ABL, and TEL-PDGFR fusion proteins. Blood 90:4947–4952
Buchdunger E, Cioffi CL, Law N, et al (2000) Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther 295:139–145
Dewar AL, Doherty KV, Hughes TP, Lyons AB (2005) Imatinib inhibits the functional capacity of cultured human monocytes. Immunol Cell Biol 83:48–56
Dewar AL, Farrugia AN, Condina MR, et al (2006) Imatinib as a potential anti-resorptive therapy for bone disease. Blood
Peng B, Hayes M, Resta D, et al (2004) Pharmacokinetics and pharmacodynamics of imatinib in a phase I trial with chronic myeloid leukemia patients. J Clin Oncol 22:935–942
Murray LJ, Abrams TJ, Long KR, et al (2003) SU11248 inhibits tumor growth and CSF-1R-dependent osteolysis in an experimental breast cancer bone metastasis model. Clin Exp Metastasis 20:757–766
Lev DC, Kim SJ, Onn A, et al (2005) Inhibition of platelet-derived growth factor receptor signaling restricts the growth of human breast cancer in the bone of nude mice. Clin Cancer Res 11:306–314
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gallet, M., Mentaverri, R., Sévenet, N. et al. Ability of breast cancer cell lines to stimulate bone resorbing activity of mature osteoclasts correlates with an anti-apoptotic effect mediated by macrophage colony stimulating factor. Apoptosis 11, 1909–1921 (2006). https://doi.org/10.1007/s10495-006-9507-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-006-9507-z