Abstract
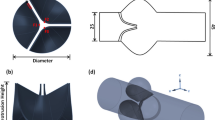
Hemodynamic stresses are presumed to play an important role in the development of calcific aortic valve disease (CAVD). The elucidation of the shear stress mechanisms involved in the pathogenesis of CAVD has been hampered by the complexity of the native unsteady and side-specific valvular flow environment. To address this gap, this article describes the design and validation of a novel device to expose leaflet samples to time-dependent side-specific shear stress. The device built on a double cone-and-plate geometry was dimensioned based on our previous single-sided shear stress device that minimizes secondary flow effects inherent to this geometry. A fluid–structure interaction (FSI) model was designed to predict the actual shear stress produced on a tissue sample mounted in the new device. Staining was performed on porcine leaflets conditioned in the new bioreactor to assess endothelial integrity and cellular apoptosis. The FSI results demonstrated good agreement between the target (native) and the actual side-specific shear stress produced on a tissue sample. No significant difference in endothelial integrity and cellular apoptosis was detected between samples conditioned for 96 h and fresh controls. This new device will enable the investigation of valvular response to normal and pathologic hemodynamics and the potential mechano-etiology of CAVD.








Similar content being viewed by others
References
Balachandran, K., S. Konduri, P. Sucosky, H. Jo, and A. P. Yoganathan. An ex vivo study of the biological properties of porcine aortic valves in response to circumferential cyclic stretch. Ann. Biomed. Eng. 34:1655–1665, 2006.
Beppu, S., S. Suzuki, H. Matsuda, F. Ohmori, S. Nagata, and K. Miyatake. Rapidity of progression of aortic stenosis in patients with congenital bicuspid aortic valves. Am. J. Cardiol. 71:322–327, 1993.
Blackman, B. R., K. A. Barbee, and L. E. Thibault. In vitro cell shearing device to investigate the dynamic response of cells in a controlled hydrodynamic environment. Ann. Biomed. Eng. 28:363–372, 2000.
Blackman, B. R., G. Garcia-Cardena, and M. A. Gimbrone, Jr. A new in vitro model to evaluate differential responses of endothelial cells to simulated arterial shear stress waveforms. J. Biomech. Eng. 124:397–407, 2002.
Breen, L. T., P. E. McHugh, B. A. McCormack, G. Muir, N. J. Quinlan, K. B. Heraty, and B. P. Murphy. Development of a novel bioreactor to apply shear stress and tensile strain simultaneously to cell monolayers. Rev. Sci. Instrum. 77:104301, 2006.
Brewer, R. J., R. M. Mentzer, Jr., J. D. Deck, R. C. Ritter, J. S. Trefil, and S. P. Nolan. An in vivo study of the dimensional changes of the aortic valve leaflets during the cardiac cycle. J. Thorac. Cardiovasc. Surg. 74:645–650, 1977.
Buschmann, M. H., P. Dieterich, and N. A. Adams. Analysis of flow in cone-and-plate apparatus with respect to spatial and temporal effects on endothelial cells. Biotechnol. Bioeng. 89:493–502, 2004.
Bussolari, S. R., C. F. Dewey, Jr., and M. A. Gimbrone, Jr. Apparatus for subjecting living cells to fluid shear stress. Rev. Sci. Instrum. 53:1851–1854, 1982.
Butcher, J. T., A. M. Penrod, A. J. Garcia, and R. M. Nerem. Unique morphology and focal adhesion development of valvular endothelial cells in static and fluid flow environments. Arterioscler. Thromb. Vasc. Biol. 24:1429–1434, 2004.
Butcher, J. T., C. A. Simmons, and J. N. Warnock. Mechanobiology of the aortic heart valve. J. Heart Valve Dis. 17:62–73, 2008.
Cacciola, G., G. W. Peters, and P. J. Schreurs. A three-dimensional mechanical analysis of a stentless fibre-reinforced aortic valve prosthesis. J. Biomech. 33:521–530, 2000.
Chambers, J. B. Aortic stenosis. Eur. J. Echocardiogr. 10:i11–i19, 2009.
Chung, C. A., M. R. Tzou, and R. W. Ho. Oscillatory flow in a cone-and-plate bioreactor. J. Biomech. Eng. 127:601–610, 2005.
Dai, G., S. Natarajan, Y. Zhang, S. Vaughn, B. R. Blackman, R. D. Kamm, G. Garcia-Cardena, and M. A. Gimbrone, Jr. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc. Natl Acad. Sci. 101:14871–14876, 2004.
De Hart, J., F. P. Baaijens, G. W. Peters, and P. J. Schreurs. A computational fluid-structure interaction analysis of a fiber-reinforced stentless aortic valve. J. Biomech. 36:699–712, 2003.
De Hart, J., G. W. M. Peters, P. J. G. Schreurs, and F. P. T. Baaijens. A two-dimensional fluid-structure interaction model of the aortic valve. J. Biomech. 33:1079–1088, 2000.
De Hart, J., G. W. M. Peters, P. J. G. Schreurs, and F. P. T. Baaijens. A three-dimensional computational analysis of fluid-structure interaction in the aortic valve. J. Biomech. 36:103–112, 2003.
Deck, J. D. Endothelial cell orientation on aortic valve leaflets. Cardiovasc. Res. 20:760–767, 1986.
Dewey, C. F., Jr., S. R. Bussolari, M. A. Gimbrone, Jr., and P. F. Davies. The dynamic response of vascular endothelial cells to fluid shear stress. J. Biomech. Eng. 103:177–185, 1981.
Donea, J., S. Guiliani, and J. P. Halleux. An arbitrary Lagrangian-Eulerian finite-element method for transient dynamic fluid structure interactions. Comput. Methods Appl. Mech. Eng. 33:689–723, 1982.
Fewell, M. E., and J. D. Hellums. The secondary flow of Newtonian fluids in cone-and-plate viscometers. Trans. Soc. Rheol. 21:535–565, 1977.
Ge, L., and F. Sotiropoulos. Direction and magnitude of blood flow shear stresses on the leaflets of aortic valves: is there a link with valve calcification? J. Biomech. Eng. 132:014505, 2010.
Go, Y. M., R. P. Patel, M. C. Maland, H. Park, J. S. Beckman, V. M. Darley-Usmar, and H. Jo. Evidence for peroxynitrite as a signaling molecule in flow-dependent activation of c-Jun NH(2)-terminal kinase. Am. J. Physiol. 277:H1647–H1653, 1999.
Haj-Ali, R., L. P. Dasi, H. S. Kim, J. Choi, H. W. Leo, and A. P. Yoganathan. Structural simulations of prosthetic tri-leaflet aortic heart valves. J. Biomech. 41:1510–1519, 2008.
Hajra, L., A. I. Evans, M. Chen, S. J. Hyduk, T. Collins, and M. I. Cybulsky. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc. Natl Acad. Sci. USA 97:9052–9057, 2000.
Jo, H., H. Song, and A. Mowbray. Role of NADPH oxidases in disturbed flow- and BMP4- induced inflammation and atherosclerosis. Antioxidants Redox Signal. 8:1609–1619, 2006.
Kadem, L., J. G. Dumesnil, R. Rieu, L. G. Durand, D. Garcia, and P. Pibarot. Impact of systemic hypertension on the assessment of aortic stenosis. Heart 91:354–361, 2005.
Kaden, J. J., and D. Haghi. Hypertension in aortic valve stenosis—a Trojan horse. Eur. Heart J. 29:1934–1935, 2008.
Kilner, P. J., G. Z. Yang, A. J. Wilkes, R. H. Mohiaddin, D. N. Firmin, and M. H. Yacoub. Asymmetric redirection of flow through the heart. Nature 404:759–761, 2000.
Ku, C. H., P. H. Johnson, P. Batten, P. Sarathchandra, R. C. Chambers, P. M. Taylor, M. H. Yacoub, and A. H. Chester. Collagen synthesis by mesenchymal stem cells and aortic valve interstitial cells in response to mechanical stretch. Cardiovasc. Res. 71:548–556, 2006.
Leo, H. L., L. P. Dasi, J. Carberry, H. A. Simon, and A. P. Yoganathan. Fluid dynamic assessment of three polymeric heart valves using particle image velocimetry. Ann. Biomed. Eng. 34:936–952, 2006.
Leo, H. L., H. Simon, J. Carberry, S. C. Lee, and A. P. Yoganathan. A comparison of flow field structures of two tri-leaflet polymeric heart valves. Ann. Biomed. Eng. 33:429–443, 2005.
Lim, W. L., Y. T. Chew, T. C. Chew, and H. T. Low. Pulsatile flow studies of a porcine bioprosthetic aortic valve in vitro: PIV measurements and shear-induced blood damage. J. Biomech. 34:1417–1427, 2001.
Mascherbauer, J., C. Fuchs, M. Stoiber, H. Schima, E. Pernicka, G. Maurer, and H. Baumgartner. Systemic pressure does not directly affect pressure gradient and valve area estimates in aortic stenosis in vitro. Eur. Heart J. 29:2049–2057, 2008.
Merryman, W. D. Mechano-potential etiologies of aortic valve disease. J. Biomech. 43:87–92, 2010.
Mooney, M., and R. H. Ewart. The conicylindrical viscometer. Physics 5:350–354; 350, 1934.
Nkomo, V. T., J. M. Gardin, T. N. Skelton, J. S. Gottdiener, C. G. Scott, and M. Enriquez-Sarano. Burden of valvular heart diseases: a population-based study. Lancet 368:1005–1011, 2006.
Otto, C. M. Valvular aortic stenosis: disease severity and timing of intervention. J. Am. Coll. Cardiol. 47:2141–2151, 2006.
Otto, C. M., J. Kuusisto, and D. D. Reichenbach. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation 90:844–853, 1994.
Pelech, I., and A. H. Shapiro. Flexible disk rotating on a gas film next to a wall. J. Appl. Mech. 31:577–584, 1964.
Platt, M. O., Y. Xing, H. Jo, and A. P. Yoganathan. Cyclic pressure and shear stress regulate matrix metalloproteinases and cathepsin activity in porcine aortic valves. J. Heart Valve Dis. 15:622–629, 2006.
Rabkin, S. W. The association of hypertension and aortic valve sclerosis. Blood Press. 14:264–272, 2005.
Rajamannan, N. M., M. Subramaniam, D. Rickard, S. R. Stock, J. Donovan, M. Springett, T. Orszulak, D. A. Fullerton, A. J. Tajik, R. O. Bonow, and T. Spelsberg. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation 107:2181–2184, 2003.
Sdougos, H. P., S. R. Bussolari, and C. F. Dewey. Secondary flow and turbulence in a cone-and-plate device. J. Fluid Mech. 138:379–404, 1984.
Sotiropoulos, F., and I. Borazjani. A review of state-of-the-art numerical methods for simulating flow through mechanical heart valves. Med. Biol. Eng. Comput. 47:245–256, 2009.
Strickberger, S. A., S. P. Schulman, and G. M. Hutchins. Association of Paget’s disease of bone with calcific aortic valve disease. Am. J. Med. 82:953–956, 1987.
Sucosky, P., K. Balachandran, A. Elhammali, H. Jo, and A. P. Yoganathan. Altered shear stress stimulates upregulation of endothelial VCAM-1 and ICAM-1 in a BMP-4- and TGF-beta1-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 29:254–260, 2009.
Sucosky, P., M. Padala, A. Elhammali, K. Balachandran, H. Jo, and A. P. Yoganathan. Design of an ex vivo culture system to investigate the effects of shear stress on cardiovascular tissue. J. Biomech. Eng. 130:035001-1–035001-8, 2008.
Thubrikar, M., S. P. Nolan, L. P. Bosher, and J. D. Deck. The cyclic changes and structure of the base of the aortic valve. Am. Heart J. 99:217–224, 1980.
Thubrikar, M., W. C. Piepgrass, L. P. Bosher, and S. P. Nolan. The elastic modulus of canine aortic valve leaflets in vivo and in vitro. Circ. Res. 47:792–800, 1980.
Weinberg, E. J., and M. R. Kaazempur Mofrad. A multiscale computational comparison of the bicuspid and tricuspid aortic valves in relation to calcific aortic stenosis. J. Biomech. 41:3482–3487, 2008.
Weston, M. W., D. V. LaBorde, and A. P. Yoganathan. Estimation of the shear stress on the surface of an aortic valve leaflet. Ann. Biomed. Eng. 27:572–579, 1999.
Weston, M. W., and A. P. Yoganathan. Biosynthetic activity in heart valve leaflets in response to in vitro flow environments. Ann. Biomed. Eng. 29:752–763, 2001.
Xing, Y., J. N. Warnock, Z. He, S. L. Hilbert, and A. P. Yoganathan. Cyclic pressure affects the biological properties of porcine aortic valve leaflets in a magnitude- and frequency-dependent manner. Ann. Biomed. Eng. 32:1461–1470, 2004.
Acknowledgments
The authors would like to thank Dr. Fotis Sotiropoulos and Dr. Liang Ge (University of Minnesota, Minneapolis, MN) for providing the shear stress data of their computational fluid dynamic aortic valve model; Dr. Santanu Chandra (University of Notre Dame, Notre Dame, IN) for his feedback; and Martin’s Custom Butchering (Wakarusa, IN) for supplying porcine hearts for this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jane Grande-Allen oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Sun, L., Rajamannan, N.M. & Sucosky, P. Design and Validation of a Novel Bioreactor to Subject Aortic Valve Leaflets to Side-Specific Shear Stress. Ann Biomed Eng 39, 2174–2185 (2011). https://doi.org/10.1007/s10439-011-0305-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-011-0305-6