Abstract
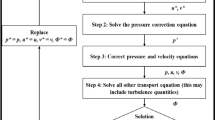
The purpose of this article is to validate numerical simulations of flow and pressure incorporating deformable walls using in vitro flow phantoms under physiological flow and pressure conditions. We constructed two deformable flow phantoms mimicking a normal and a restricted thoracic aorta, and used a Windkessel model at the outlet boundary. We acquired flow and pressure data in the phantom while it operated under physiological conditions. Next, in silico numerical simulations were performed, and velocities, flows, and pressures in the in silico simulations were compared to those measured in the in vitro phantoms. The experimental measurements and simulated results of pressure and flow waveform shapes and magnitudes compared favorably at all of the different measurement locations in the two deformable phantoms. The average difference between measured and simulated flow and pressure was approximately 3.5 cc/s (13% of mean) and 1.5 mmHg (1.8% of mean), respectively. Velocity patterns also showed good qualitative agreement between experiment and simulation especially in regions with less complex flow patterns. We demonstrated the capabilities of numerical simulations incorporating deformable walls to capture both the vessel wall motion and wave propagation by accurately predicting the changes in the flow and pressure waveforms at various locations down the length of the deformable flow phantoms.









Similar content being viewed by others
References
Acevedo-Bolton, G., L. D. Jou, B. P. Dispensa, M. T. Lawton, R. T. Higashida, A. J. Martin, et al. Estimating the hemodynamic impact of interventional treatments of aneurysms: numerical simulation with experimental validation: technical case report. Neurosurgery 59(2):E429–E430, 2006; (author reply E-30).
Anderson, J., H. G. Wood, P. E. Allaire, and D. B. Olsen. Numerical analysis of blood flow in the clearance regions of a continuous flow artificial heart pump. Artif. Organs 24(6):492–500, 2000.
Arcaute, K., and R. Wicker. Patient-specific compliant vessel manufacturing using dip-spin coating of rapid prototyped molds. J. Manuf. Sci. Eng. 130:051008, 2008.
Benard, N., R. Perrault, and D. Coisne. Blood flow in stented coronary artery: numerical fluid dynamics analysis. Conf. Proc. IEEE Eng. Med. Biol. Soc. 5:3800–3803, 2004.
Cortez, M., R. Quintana, and R. Wicker. Multi-step dip-spin coating manufacturing system for silicone cardiovascular membrane fabrication with prescribed compliance. Int. J. Adv. Manuf. Technol. 34(7):667–679, 2007.
Dobrin, P. Mechanical properties of arteries. Physiol. Rev. 58(2):397, 1978.
Figueroa, C. A., and J. P. Ku. SimVascular. https://simtk.org/home/simvascular. Accessed 1 September 2010.
Figueroa, C. A., I. E. Vignon-Clementel, K. E. Jansen, T. J. R. Hughes, and C. A. Taylor. A coupled momentum method for modeling blood flow in three-dimensional deformable arteries. Comput. Methods Appl. Mech. Eng. 195(41–43):5685–5706, 2006.
Glagov, S., C. Zarins, D. P. Giddens, and D. N. Ku. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch. Pathol. Lab. Med. 112(10):1018–1031, 1988.
Goergen, C., B. Johnson, J. Greve, C. Taylor, and C. Zarins. Increased anterior abdominal aortic wall motion: possible role in aneurysm pathogenesis and design of endovascular devices. J. Endovasc. Ther. 14(4):574–584, 2007.
Grant, B. J., and L. J. Paradowski. Characterization of pulmonary arterial input impedance with lumped parameter models. Am. J. Physiol. 252(3 Pt 2):H585–H593, 1987.
Kim, H. J., C. A. Figueroa, T. J. R. Hughes, K. E. Jansen, and C. A. Taylor. Augmented Lagrangian method for constraining the shape of velocity profiles at outlet boundaries for three-dimensional finite element simulations of blood flow. Comput. Methods Appl. Mech. Eng. 198:3551–3566, 2009.
Hager, A., H. Kaemmerer, U. Rapp-Bernhardt, S. Blucher, K. Rapp, T. Bernhardt, et al. Diameters of the thoracic aorta throughout life as measured with helical computed tomography. J. Thorac. Cardiovasc. Surg. 123(6):1060, 2002.
Hoi, Y., S. H. Woodward, M. Kim, D. B. Taulbee, and H. Meng. Validation of CFD simulations of cerebral aneurysms with implication of geometric variations. J. Biomech. Eng. 128(6):844–851, 2006.
Jou, L. D., C. M. Quick, W. L. Young, M. T. Lawton, R. Higashida, A. Martin, et al. Computational approach to quantifying hemodynamic forces in giant cerebral aneurysms. AJNR Am. J. Neuroradiol. 24(9):1804–1810, 2003.
Ku, J. P., C. J. Elkins, and C. A. Taylor. Comparison of CFD and MRI flow and velocities in an in vitro large artery bypass graft model. Ann. Biomed. Eng. 33(3):257–269, 2005.
Kung, E., and C. Taylor. Development of a physical Windkessel module to re-create in vivo vascular flow impedance for in vitro experiments. Cardiovasc. Eng. Technol. 2:2–14, 2010.
Kung, E. O., A. S. Les, F. Medina, R. Wicker, M. McConnell, and C. A. Taylor. In vitro validation of finite element model of AAA hemodynamics incorporating realistic outflow boundary conditions. J. Biomech. Eng. 133(4):041003, 2011. doi:10.1115/1.4003526.
Li, Z., and C. Kleinstreuer. Blood flow and structure interactions in a stented abdominal aortic aneurysm model. Med. Eng. Phys. 27(5):369–382, 2005.
Lotz, J., C. Meier, A. Leppert, and M. Galanski. Cardiovascular flow measurement with phase-contrast MR imaging: basic facts and implementation. Radiographics 22(3):651–671, 2002.
Love, A. A Treatise on the Mathematical Theory of Elasticity. New York: Dover Publications, 1944.
McCauley, T. R., C. S. Pena, C. K. Holland, T. B. Price, and J. C. Gore. Validation of volume flow measurements with cine phase-contrast MR imaging for peripheral arterial waveforms. J. Magn. Reson. Imaging 5(6):663–668, 1995.
Migliavacca, F., R. Balossino, G. Pennati, G. Dubini, T. Y. Hsia, M. R. de Leval, et al. Multiscale modelling in biofluidynamics: application to reconstructive paediatric cardiac surgery. J. Biomech. 39(6):1010–1020, 2006.
Mills, C., I. Gabe, J. Gault, D. Mason, J. Ross, Jr., E. Braunwald, et al. Pressure-flow relationships and vascular impedance in man. Cardiovasc. Res. 4(4):405–417, 1970.
Mohiaddin, R., P. Kilner, S. Rees, and D. Longmore. Magnetic resonance volume flow and jet velocity mapping in aortic coarctation. J. Am. Coll. Cardiol. 22(5):1515, 1993.
O’Rourke, M., R. Kelly, and A. Avolio. The Arterial Pulse. Philadelphia: Lea and Febiger, pp. 15–20, 1992.
Olufsen, M. S. A one-dimensional fluid dynamic model of the systemic arteries. Stud. Health Technol. Inform. 71:79–97, 2000.
Pearson, A., R. Guo, D. Orsinelli, P. Binkley, and T. Pasierski. Transesophageal echocardiographic assessment of the effects of age, gender, and hypertension on thoracic aortic wall size, thickness, and stiffness. Am. Heart J. 128(2):344–351, 1994.
Remington, J., and E. Wood. Formation of peripheral pulse contour in man. J. Appl. Physiol. 9(3):433, 1956.
Segers, P., S. Brimioulle, N. Stergiopulos, N. Westerhof, R. Naeije, M. Maggiorini, et al. Pulmonary arterial compliance in dogs and pigs: the three-element Windkessel model revisited. Am. J. Physiol. 277(2 Pt 2):H725–H731, 1999.
Shipkowitz, T., V. Rodgers, L. Frazin, and K. Chandran. Numerical study on the effect of secondary flow in the human aorta on local shear stresses in abdominal aortic branches. J. Biomech. 33(6):717–728, 2000.
Tai, N., H. Salacinski, A. Edwards, G. Hamilton, and A. Seifalian. Compliance properties of conduits used in vascular reconstruction. Br. J. Surg. 87(11):1516–1524, 2000.
Taylor, C. A., M. T. Draney, J. P. Ku, D. Parker, B. N. Steele, K. Wang, et al. Predictive medicine: computational techniques in therapeutic decision-making. Comput. Aided Surg. 4(5):231–247, 1999.
Taylor, C. A., T. J. Hughes, and C. K. Zarins. Finite element modeling of three-dimensional pulsatile flow in the abdominal aorta: relevance to atherosclerosis. Ann. Biomed. Eng. 26(6):975–987, 1998.
Taylor, C. A., and D. A. Steinman. Image-based modeling of blood flow and vessel wall dynamics: applications, methods and future directions. Sixth International Bio-Fluid Mechanics Symposium and Workshop, Pasadena, CA, March 28–30, 2008.
Vignon-Clementel, I. E., C. A. Figueroa, K. E. Jansen, and C. A. Taylor. Outflow boundary conditions for three-dimensional finite element modeling of blood flow and pressure in arteries. Comput. Methods Appl. Mech. Eng. 195(29–32):3776–3796, 2006.
Wentzel, J. J., F. J. Gijsen, J. C. Schuurbiers, R. Krams, P. W. Serruys, P. J. De Feyter, et al. Geometry guided data averaging enables the interpretation of shear stress related plaque development in human coronary arteries. J. Biomech. 38(7):1551–1555, 2005.
Westerhof, N., G. Elzinga, and P. Sipkema. An artificial arterial system for pumping hearts. J. Appl. Physiol. 31(5):776–781, 1971.
Westerhof, N., J. W. Lankhaar, and B. E. Westerhof. The arterial Windkessel. Med. Biol. Eng. Comput. 47(2):131–141, 2009.
Weydahl, E., and J. Moore. Dynamic curvature strongly affects wall shear rates in a coronary artery bifurcation model. J. Biomech. 34(9):1189–1196, 2001.
Acknowledgments
The authors would like to thank Lakhbir Johal, Chris Elkins, Sandra Rodriguez, Anne Sawyer, and all staff at the Lucas Center at Stanford University for assistance with the imaging experiments. This work was supported by the National Institutes of Health (Grants P50 HL083800, P41 RR09784, and U54 GM072970) and the National Science Foundation (0205741, and CNS-0619926 for computer resources).
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Joan Greve oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Kung, E.O., Les, A.S., Figueroa, C.A. et al. In Vitro Validation of Finite Element Analysis of Blood Flow in Deformable Models. Ann Biomed Eng 39, 1947–1960 (2011). https://doi.org/10.1007/s10439-011-0284-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-011-0284-7