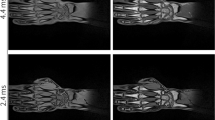
Abstract
In recent years, development of rheumatoid arthritis (RA) drug therapy has been more directly targeted to counteract specific mechanisms of inflammation, and it is now believed that early aggressive treatment with disease modifying drugs is important to inhibit future structural joint damage. The development of these new treatments has increased the need for methodologies to assess disease activity in RA and monitor the effectiveness of drug therapy. Unlike X-ray, which shows only structural bone damage, magnetic resonance imaging (MRI) can depict soft tissue damage and synovitis, the primary pathology of RA. Recent studies have also indicated that MRI is sensitive to pathophysiologic changes that may predate radiographic erosions and may predict future joint damage. In this study, we have developed a computer automated analysis technique for MR wrist images that provides an objective measure of RA synovitis. This method applies a two-compartment pharmacokinetic model to every voxel of a dynamic contrast-enhanced MRI (DCE-MRI) dataset and outputs resulting parametric images. The aim of this technique is to not only objectively quantify the severity of rheumatoid synovitis, but to also locally determine where areas of serious disease activity are situated through kinetic modeling of blood-tissue exchange. Preliminary results show good correlation to early enhancement rate, which has previously been shown to be a useful clinical marker of RA activity. However, the use of tracer kinetic modeling methods potentially provides more specific information regarding underlying RA physiology. This approach could provide a useful new tool in RA patient management and could substantially improve RA therapeutic studies by calculating objective biomarkers of the disease state.






Similar content being viewed by others
References
Bertoldo A., Vicini P., Sambuceti G., Lammertsma A. A., Parodi O., Cobelli C. 1998 Evaluation of compartmental and spectral analysis models of [18F]FDG kinetics for heart and brain studies with PET. IEEE Trans. Biomed. Eng. 45(12):1429–1448
Boers M., A. C. Verhoeven, H. M. Markusse, M. A. van de Laar, R. Westhovens, J. C. van Denderen, D. van Zeben, B. A. Dijkmans, A. J. Peeters, P. Jacobs, H. R. van den Brink, H. J. Schouten, D. M. van der Heijde, A. Boonen, and S. van der Linden. Randomised comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet 350(9074):309–318, 1997. Erratum in: Lancet 351(9097):220, 1998
Cimmino M. A., et al. 2003 Dynamic gadolinium-enhanced magnetic resonance imaging of the wrist in patients with rheumatoid arthritis can discriminate active from inactive disease. Arthritis Rheum. 48(5):1207–1213
Conaghan P. G., O’Connor P., McGonagle D., Astin P., Wakefield R. J., Gibbon W. W., Quinn M., Karim Z., Green M. J., Proudman S., Isaacs J., Emery P. 2003 Elucidation of the relationship between synovitis and bone damage: a randomized magnetic resonance imaging study of individual joints in patients with early rheumatoid arthritis. Arthritis Rheum. 48(1):64–71
Crone C. 1963 The permeability of capillaries in various organs as determined by use of the ‘indicator diffusion’ method Acta Physiol. Scand. 58:292–305
Daldrup H., Shames D. M., Wendland M., Okuhata Y., Link T. M., Rosenau W., Lu Y., Brasch R. C. 1998 Correlation of dynamic contrast-enhanced magnetic resonance imaging with histologic tumor grade: comparison of macromolecular and small-molecular contrast media Pediatr. Radiol. 28(2):67–78
Daldrup H. E., Shames D. M., Husseini W., Wendland M. F., Okuhata Y., Brasch R. C. 1998 Quantification of the extraction fraction for gadopentetate across breast cancer capillaries Magn. Reson. Med. 40(4):537–543
Daldrup-Link H. E., J. Rydland, T. H. Helbich, A. Bjornerud, K. Turetschek, K. A. Kvistad, E. Kaindl, T. M. Link, K. Staudacher, D. Shames, R. C. Brasch, O. Haraldseth, and E. J. Rummeny. Quantification of breast tumor microvascular permeability with feruglose-enhanced M.R. imaging: initial phase II multicenter trial. Radiology 229(3):885–892, 2003. Epub 2003 Oct 23
Felson D. T., Anderson J. J., Boers M., Bombardier C., Furst D., Goldsmith C., Katz L. M., Lightfoot R. Jr, Paulus H., Strand V., et al 1995 American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis Arthritis Rheum. 38(6):727–735
Fiocco U., Ferro F., Cozzi L., Vezzu M., Sfriso P., Checchetto C., Bianchi F. C., Nardacchione R., Piccoli A., Todesco S., Rubaltelli L. 2003 Contrast medium in power Doppler ultrasound for assessment of synovial vascularity: comparison with arthroscopy J. Rheumatol. 30(10):2170–2176
Foster D. M. 1998 Developing and testing integrated multicompartment models to describe a single-input multiple-output study using the SAAM II software system Adv. Exp. Med. Biol. 445:59–78
Gaffney K., Cookson J., Blades S., Coumbe A., Blake D. 1998 Quantitative assessment of the rheumatoid synovial microvascular bed by gadolinium-DTPA enhanced magnetic resonance imaging Ann. Rheum. Dis. 57(3):152–157
Gardner J. C., Zierhut M. L., Gardner G. C., Mease P., Sharp J. T., Spilker M. E., Vicini P. 2003 Changes in MRI measures of Synovial Vascularity in the RA wrist at 1–2 months following Initiation of anti-TNFα and Methotrexate Therapy Arthritis Rheum. 48(9):S131
Gardner J. C., M. L. Zierhut, J. T. Sharp, G. C. Gardner, and P. Mease. 2005 Quantitative dynamic contrast-enhanced (DCE-MRI) wrist imaging in RA correlates with changes in clinical measures following initiation of anti-TNFa and MTX therapy: a novel analytic approach. Arthritis Rheum., 52(9):S109–S110
Hayes C. E., Mathis C. M., Yuan C. 1996 Surface coil phased arrays for high-resolution imaging of the carotid arteries J. Magn. Reson. Imaging 6(1):109–112
Jevtic V., Watt I., Rozman B., Kos-Golja M., Rupenovic S., Logar D., Presetnik M., Jarh O., Demsar F., Musikic P., et al 1993 Precontrast and postcontrast (Gd-DTPA) magnetic resonance imaging of hand joints in patients with rheumatoid arthritis Clin. Radiol. 48(3):176–181
Johnson J. A., Wilson T. A. 1966 A model for capillary exchange Am. J. Physiol. 210(6):1299–1303
Kenney J., Schmiedl U., Maravilla K., Starr F., Graham M., Spence A., Nelson J. 1992 Measurement of blood-brain barrier permeability in a tumor model using magnetic resonance imaging with gadolinium-DTPA Magn. Reson. Med. 27(1):68–75
Kerwin W., Hooker A., Spilker M., Vicini P., Ferguson M., Hatsukami T., Yuan C. 2003 Quantitative magnetic resonance imaging analysis of neovasculature volume in carotid atherosclerotic plaque Circulation 107(6):851–856
Konig H., Sieper J., Wolf K. J. 1990 Rheumatoid arthritis: evaluation of hypervascular and fibrous pannus with dynamic MR imaging enhanced with Gd-DTPA Radiology 176(2):473–477
Landewe R. B., Boers M., Verhoeven A. C., Westhovens R., van de Laar M. A., Markusse H. M., van Denderen J. C., Westedt M. L., Peeters A. J., Dijkmans B. A., Jacobs P., Boonen A., van der Heijde D. M., van der Linden S. 2002 COBRA combination therapy in patients with early rheumatoid arthritis: long-term structural benefits of a brief intervention Arthritis Rheum. 46(2):347–356
Li K. L., Zhu X. P., Jackson A. 2000 Parametric mapping of scaled fitting error in dynamic susceptibility contrast enhanced MR perfusion imaging Br. J. Radiol. 73(869):470–481
McQueen F. M., Benton N., Crabbe J., Robinson E., Yeoman S., McLean L., Stewart N. 2001 What is the fate of erosions in early rheumatoid arthritis? Tracking individual lesions using X-rays and magnetic resonance imaging over the first two years of disease Ann. Rheum. Dis. 60(9):859–868
Middleton J., Americh L., Gayon R., Julien D., Aguilar L., Amalric F., Girard J. P. 2004 Endothelial cell phenotypes in the rheumatoid synovium: activated, angiogenic, apoptotic and leaky. Arthritis Res. Ther. 6(2):60–72
Mottonen T., P. Hannonen, M. Korpela, M. Nissila, H. Kautiainen, J. Ilonen, L. Laasonen, O. Kaipiainen-Seppanen, P. Franzen, T. Helve, J. Koski, M. Gripenberg-Gahmberg, R. Myllykangas-Luosujarvi, M. Leirisalo-Repo for the FIN-RACo Trial Group Delay to institution of therapy and induction of remission using single-drug or combination-disease-modifying antirheumatic drug therapy in early rheumatoid arthritis. Arthritis Rheum. 46(4):894–498, 2002.
Mottonen T., Hannonen P., Leirisalo-Repo M., Nissila M., Kautiainen H., Korpela M., Laasonen L., Julkunen H., Luukkainen R., Vuori K., Paimela L., Blafield H., Hakala M., Ilva K., Yli-Kerttula U., Puolakka K., Jarvinen P., Hakola M., Piirainen H., Ahonen J., Palvimaki I., Forsberg S., Koota K., Friman C. 1999 Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis: a randomised trial. FIN-RACo trial group. Lancet 353(9164):1568–1573
Nosas-Garcia S., Moehler T., Wasser K., Kiessling F., Bartl R., Zuna I., Hillengass J., Goldschmidt H., Kauczor H. U., Delorme S. 2005 Dynamic contrast-enhanced MRI for assessing the disease activity of multiple myeloma: a comparative study with histology and clinical markers. J. Magn. Reson. Imaging 22(1): 154–162
Ostergaard M., Stoltenberg M., Gideon P., Sorensen K., Henriksen O., Lorenzen I. 1996 Changes in synovial membrane and joint effusion volumes after intraarticular methylprednisolone. Quantitative assessment of inflammatory and destructive changes in arthritis by MRI. J. Rheumatol. 23(7): 1151–1161
Ostergaard M., Hansen M., Stoltenberg M., Jensen K. E., Szkudlarek M., Pedersen-Zbinden B., Lorenzen I. 2003 New radiographic bone erosions in the wrists of patients with rheumatoid arthritis are detectable with magnetic resonance imaging a median of two years earlier. Arthritis Rheum. 48(8):2128–2131
Ostergaard M., Stoltenberg M., Lovgreen-Nielsen P., Volck B., Sonne-Holm S., Lorenzen I. 1998 Quantification of synovitis by MRI: correlation between dynamic and static gadolinium-enhanced magnetic resonance imaging and microscopic and macroscopic signs of synovial inflammation. Magn. Reson. Imaging 16(7):743–754
Patlak C. S., Blasberg R. G., Fenstermacher J. D. 1983 Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J. Cereb. Blood Flow Metab. 3(1):1–7
Price J. C. 2003 Principles of tracer kinetic analysis. Neuroimaging Clin. N. Am. 13(4):689–704
Reece R. J., et al 2002 Comparative assessment of leflunomide and methotrexate for the treatment of rheumatoid arthritis, by dynamic enhanced magnetic resonance imaging. Arthritis Rheum. 46(2):366–372
Renkin E. M. 1959 Transport of potassium-42 from blood to tissue in isolated mammalian skeletal muscles. Am. J. Physiol. 197:1205–1210
Roberts, C., Issa B., Stone A., Jackson A., Waterton J. C., Parker G. J. 2006 Comparative study into the robustness of compartmental modeling and model-free analysis in DCE-MRI studies. J. Magn. Reson. Imaging 23(4):554–563
Roberts T. P. 1997 Physiologic measurements by contrast-enhanced MR imaging: expectations and limitations. J. Magn. Reson. Imaging 7(1):82–90
Shames D. M., et al 1993 Measurement of capillary permeability to macromolecules by dynamic magnetic resonance imaging: a quantitative noninvasive technique. Magn. Reson. Med. 29(5):616–622
Stone M., Bergin D., Whelan B., Maher M., Murray J., McCarthy C. 2001 Power Doppler ultrasound assessment of rheumatoid hand synovitis J. Rheumatol. 28(9):1979–1982
Su M. Y., Cheung Y. C., Fruehauf J. P., Yu H., Nalcioglu O., Mechetner E., Kyshtoobayeva A., Chen S. C., Hsueh S., McLaren C. E., Wan Y. L. 2003 Correlation of dynamic contrast enhancement MRI parameters with microvessel density and VEGF for assessment of angiogenesis in breast cancer J. Magn. Reson. Imaging 18(4):467–477
Tofts P. S., Brix G., Buckley D. L., Evelhoch J. L., Henderson E., Knopp M. V., Larsson H. B., Lee T. Y., Mayr N. A., Parker G. J., Port R. E., Taylor J., Weisskoff R. M. 1999 Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols J. Magn. Reson. Imaging 10(3):223–232
van der Heijde D., Dankert T., Nieman F., Rau R., Boers M. 1999 Reliability and sensitivity to change of a simplification of the Sharp/van der Heijde radiological assessment in rheumatoid arthritis Rheumatology (Oxford) 38(10):941–947
van Dijke C. F., et al 1999 MR imaging of the arthritic rabbit knee joint using albumin-(Gd-DTPA)30 with correlation to histopathology Magn. Reson. Imaging 17(2):237–45
Workie D. W., Dardzinski B. J. 2005 Quantifying dynamic contrast-enhanced MRI of the knee in children with juvenile rheumatoid arthritis using an arterial input function (AIF) extracted from popliteal artery enhancement, and the effect of the choice of the AIF on the kinetic parameters Magn. Reson. Med. 54(3):560–568
Yeung W. T., Lee T. Y., Del Maestro R. F., Kozak R., Brown T. 1992 In vivo CT measurement of blood-brain transfer constant of iopamidol in human brain tumors J. Neurooncol. 14(2):177–187
Acknowledgments
The authors acknowledge and thank Gregory Gardner, Philip Mease and David White for their contribution to this work. The authors also wish to thank the anonymous reviewers for their comments, which resulted in a much improved manuscript. This study was supported by NIH grant P41 EB-001975, the University of Washington Royalty Research Fund and Amgen Inc.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Appendix I. Blood-Tissue Exchange Model Description and Early Enhancement Rate
In this simplified version of our special case of the Patlak method, EER is calculated as the early enhancement slope of the dynamic measurement in a simplified blood-tissue exchange model (see Fig. A1). The dynamic measurement, s(t), is calculated as the sum of the interstitial space (tissue), q 3, and the scaled vascular region, f BV· q 1, and is also represented as the change in signal intensity from pre- to post-Gd-DTPA-BMA enhancement. Specifically:
Simplified blood-tissue exchange model. Similar to Figs. 1 and 2, in this simplified model the state variable q 1 (t) represents Gd concentration (mol l−1) in the vasculature. The rate constant k 01 (s−1) describes the fractional flux of Gd leaving of the vasculature. A further simplification is that there is a bolus Gd injection instead of a 30-s infusion. The state variable q 3(t) represents Gd concentration (mol l−1) in the interstitial space. The fractional rate constant k 31 (s−1) describes the flux of Gd from vasculature into interstitial space. The amount of blood present in the tissue is represented by f BV (ml blood 100 cc−1 tissue), or fractional blood volume (fraction of q 1). The arbitrary unit, m, represents the measured value of Gd concentration in both compartments (f BV · q 1 + q 3 ( ΔSI). It should be apparent that this model is simpler than, but similar to, the model used for our analyses.
where m(t) is the post-Gd signal intensity at time t, and m(t 0) is the pre-Gd signal intensity.
The simplified blood-tissue exchange model can be represented as a set of ordinary differential equations, whose solutions can be approximated using the first order Taylor series:
The dynamic measurement is thus approximated by the first order Taylor series according to the following equation:
where q 1(t 0) and k 01 are constant for every voxel (defined by the input function). This approximation is valid for small values of t. The initial rate of Gd enhancement can be estimated as the spatially normalized constant slope of Eq. 9:
where \(\bar m(t_0 )\) is the spatial average of the baseline image and t is approximately 55 s. It is important to note that the spatially normalizing factor, \(\bar m(t_0 )\), is constant for all voxels in our case, much like the approach in previously published ROI analyses.30,3 Therefore, the only spatially varying parameters are K PS and f BV. Moreover, Eq. 10 should correlate strongly with a linear combination of the two parameters we have estimated with the Patlak method: K PS and f BV. That is,
for small values of t, where A is a proportionality constant and does not vary spatially. We think that this general conclusion based on the simplified blood-tissue exchange model may also be applicable to the more complex model used in our analyses.
Appendix II. Automated Selection of SFE Masking Threshold
To select the optimum SFE masking threshold we analyze the number of voxels included in the SFE mask for all unit SFE thresholds, from 1 to 100. The SFE threshold that causes the first slope decrease (first point of inflection) in the number of voxels is selected as the optimum SFE threshold. This point occurs where the number of voxels in the mask is most sensitive to the chosen threshold value. Figure A2 shows the sigmoid relationship between the natural logarithm of the SFE threshold and the number of voxels in the mask.
Optimal SFE masking threshold. The vertical axis shows the number of voxels included in the SFE mask. The horizontal axis shows the natural logarithm of the SFE threshold. Searching from low to high SFE thresholds, the software determines the first SFE threshold value where the slope of the curve decreases (first point of inflection). The SFE threshold value at the first point of inflection on this plot is considered the optimal SFE masking threshold, as it marks the point of maximum sensitivity of the number of voxels to the choice of the SFE threshold.
Rights and permissions
About this article
Cite this article
Zierhut, M.L., Gardner, J.C., Spilker, M.E. et al. Kinetic Modeling of Contrast-Enhanced MRI: An Automated Technique for Assessing Inflammation in the Rheumatoid Arthritis Wrist. Ann Biomed Eng 35, 781–795 (2007). https://doi.org/10.1007/s10439-006-9249-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-006-9249-7