Abstract
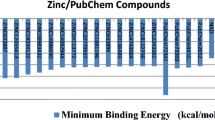
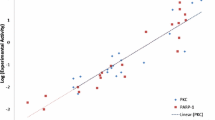
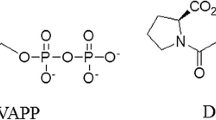
The 2C-methylerythritol 4-phosphate (MEP) pathway for the biosynthesis of isopentenyl pyrophosphate and its isomer dimethylallyl pyrophosphate, which are the precursors of isoprenoids, is present in plants, in the malaria parasite Plasmodium falciparum and in most eubacteria, including pathogenic agents. However, the MEP pathway is absent from fungi and animals, which have exclusively the mevalonic acid pathway. Given the characteristics of the MEP pathway, its enzymes represent potential targets for the generation of selective antibacterial, antimalarial and herbicidal molecules. We have focussed on the enzyme 4-(cytidine 5′-diphospho)-2-C-methyl-d-erythritol kinase (CMK), which catalyses the fourth reaction step of the MEP pathway. A molecular dynamics simulation was carried out on the CMK dimer complex, and protein–protein interactions analysed, considering also water-mediated interactions between monomers. In order to find small molecules that bind to CMK and disrupt dimer formation, interactions observed in the dynamics trajectory were used to model a pharmacophore used in database searches. Using an intensity-fading matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry approach, one compound was found to interact with CMK. The data presented here indicate that a virtual screening approach can be used to identify candidate molecules that disrupt the CMK–CMK complex. This strategy can contribute to speeding up the discovery of new antimalarial, antibacterial, and herbicidal compounds.







Similar content being viewed by others
References
Sacchettini JC, Poulter CD (1997) Science 277(5333):1788–1789
Chappell J (1995) Annu Rev Plant Physiol Plant Mol Biol 46:521–547
McGarvey DJ, Croteau R (1995) Plant Cell 7(7):1015–1026
Croteau R, Kutchan T, Lewis N (2000) Natural products (secondary metabolites). In: Buchanan B, Gruissem W, Jones R (eds) Biochemistry and molecular biology of plants. American Society of Plant Biologists, Rockville, MD, pp 1250–1268
Chappell J (2002) Curr Opin Plant Biol 5(2):151–157
Flesch G, Rohmer M (1988) Eur J Biochem 175(2):405–411
Rohmer M, Knani M, Simonin P, Sutter B, Sahm H (1993) Biochem J 295(Pt 2):517–524
Schwarz MK (1994) Terpenbiosynthese in Ginkgo biloba: Eine überraschende Geschichte. PhD Thesis. Eidgenoessische Technische Hochschule, Zurich
Rodríguez-Concepción M, Boronat A (2002) Plant Physiol 130(3):1079–1089
Rohmer M, Grosdemange-Billiard C, Seemann M, Tritsch D (2004) Curr Opin Investig Drugs 5(2):154–162
Boucher Y, Doolittle WF (2000) Mol Microbiol 37(4):703–716
Lichtenthaler HK, Schwender J, Disch A, Rohmer M (1997) FEBS Lett 400:271–274
Eisenreich W, Schwarz M, Cartayrade A, Arigoni D, Zenk MH, Bacher A (1998) Chem Biol 5(9):R221–R233
Lichtenthaler HK (1999) Annu Rev Plant Physiol Plant Mol Biol 50:47–65
Rohmer M (1999) Nat Prod Rep 16(5):565–574 (Oct)
Kuzuyama T, Shimizu T, Takahashi S, Seto H (1998) Tetrahedron Lett 39(43):7913–7916
Jomaa H, Wiesner J, Sanderbrand S, Altincicek B, Weidemeyer C, Hintz M, Turbachova I, Eberl M, Zeidler J, Lichtenthaler HK, Soldati D, Beck E (1999) Science 285(5433):1573–1576
Lichtenthaler HK (2000) Biochem Soc Trans 28:785–789
Testa CA, Brown MJ (2003) Curr Pharm Biotechnol 4(4):248–259
Wiesner J, Borrmann S, Jomaa H (2003) Parasitol Res 90(2):S71–S76
Missinou MA, Borrmann S, Schindler A, Issifou S, Adegnika AA, Matsiegui PB, Binder R, Lell B, Wiesner J, Baranek T, Jomaa H, Kremsner PG (2002) Lancet 360(9349):1941–1942
Zeidler JG, Schwender J, Müller C, Wiesner J, Weidemeyer C, Beck E, Jomaa H, Lichtenthaler HK (1998) Z Naturforsch 53(c):980–986
Borrmann S, Issifou S, Esser G, Adegnika AA, Ramharter M, Matsiegui PB, Oyakhirome S, Mawili-Mboumba DP, Missinou MA, Kun JF, Jomaa H, Kremsner PG (2004) J Infect Dis 190(9):1534–1540
Miallau L, Alphey MS, Kemp LE, Leonard GA, McSweeney SM, Hecht S, Bacher A, Eisenreich W, Rohdich F, Hunter WN (2003) Proc Natl Acad Sci USA 100(16):9173–9178
Gabrielsen M, Bond CS, Hallyburton I, Hecht S, Bacher A, Eisenreich W, Rohdich F, Hunter WN (2004) J Biol Chem 279(50):52753–52761
Wells JA, McClendon CL (2007) Nature 450(7172):1001–1009
Sperandio O, Miteva MA, Segers K, Nicolaes GA, Villoutreix BO (2008) Open Biochem J2:29–37
Smrcka AV, Lehmann DM, Dessal AL (2008) Comb Chem High Throughput Screen 11(5):382–395
Fletcher S, Hamilton AD (2007) Curr Top Med Chem (10):922–7
Whitty A, Kumaravel G (2006) Nat Chem Biol 2(3):112–118
Samsonov S, Teyra J, Pisabarro MT (2008) Proteins 73(2):515–525
Jiang L, Kuhlman B, Kortemme T, Baker D (2005) Proteins 58(4):893–904
Furukawa Y, Morishima I (2001) J Biol Chem 20 276(16):12983–12990
Langhorst U, Backmann J, Loris R, Steyaert J (2000) Biochemistry 39(22):6586–6593
Janin J (1999) Structure 7(12):R277–R279
van Dijk AD, Bonvin AM (2006) Bioinformatics 22(19):2340–2347
Yanes O, Villanueva J, Querol E, Aviles FX (2005) Mol Cell Proteomics 4(10):1602–1613
Villanueva J, Yanes O, Querol E, Serrano L, Aviles FX (2003) Anal Chem 75(14):3385–3395
Case DA, Pearlman DA, Caldwell JW, Cheatham TEI, Wang J, Ross WS, Simmerling C, Darden T, Merz KM, Stanton RV, Cheng A, Vincent JJ, Crowley M, Tsui V, Gohlke H, Radmer R, Duan Y, Pitera J, Massova I, Seibel GL, Singh UC, Weiner P, Kollman PA (2002) AMBER7. University of California, San Francisco, CA
Wang R, Lai L, Wang S (2002) J Comput Aided Mol Des 16:11
Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA (2004) J Comput Chem 25:1157
Jakalian A, Bush BL, Jack DB, Bayly CL (2000) J Comp Chem 21(2):132–146
Jorgensen WL, Chandrasekhar J, Madura JD (1983) J Chem Phys 79:926
Darden T, York D, Pedersen L (1993) J Chem Phys 98:10089
Ryckaert JP, Ciccotti G, Berendsen HJC (1977) J Comp Phys 23:327
Berendsen HJC, Postman JPM, Van Gunsteren WF, DiNola A, Haak JR (1984) J Chem Phys 81:3684
Bernal C, Mendez E, Terencio J, Boronat A, Imperial S (2005) Anal Biochem 340(2):245–251
Bradford MM (1976) Anal Biochem 72:248–254
Karas M, Hillenkamp F (1988) Anal Chem 60(20):2299–2301
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) J Comput Chem 25(13):1605–1612
Humphrey W, Dalke A, Schulten K (1996) J Mol Graph 14(1):33–38,27–38
Levy Y, Onuchic JN (2006) Annu Rev Biophys Biomol Struct 35:389–415
Sreenivasan U, Axelsen PH (1992) Biochemistry 31(51):12785–12791
Acknowledgements
The excellent technical assistance of Drs. Eliandre de Oliveira and Maria Antonia Odena Caballol, from the Proteomics Platform, Barcelona Science Park, in the intensity-fading MALDI-TOF mass spectrometry experiments is gratefully acknowledged. This work was financed in part with grants from the University of Barcelona (ACES-UB), the Spanish Ministerio de Ciencia y Tecnología (CTQ2006-06588/BQU, BIO2002-04419-C02-02 and BIO2008-01184) and the Generalitat de Catalunya (2005SGR00914).
Author information
Authors and Affiliations
Corresponding author
Additional information
V. Giménez-Oya and Ó. Villacañas contributed equally to this work.
Rights and permissions
About this article
Cite this article
Giménez-Oya, V., Villacañas, Ó., Fernàndez-Busquets, X. et al. Mimicking direct protein–protein and solvent-mediated interactions in the CDP-methylerythritol kinase homodimer: a pharmacophore-directed virtual screening approach. J Mol Model 15, 997–1007 (2009). https://doi.org/10.1007/s00894-009-0458-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-009-0458-5