Abstract
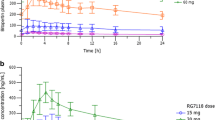
High doses of glycine have been reported to improve negative schizophrenic symptoms, suggesting that ingested glycine activates glutamatergic transmission via N-methyl-d-aspartate (NMDA) receptors. However, the pharmacokinetics of administered glycine in the brain has not been evaluated. In the present study, the time- and dose-dependent distributions of administered glycine were investigated from a pharmacokinetic viewpoint. Whole-body autoradiography of radiolabeled glycine was performed, and time–concentration curves for glycine and serine in plasma, cerebrospinal fluid (CSF), and brain tissues were obtained. Furthermore, pharmacokinetic parameters were calculated. For a more detailed analysis, the amount of glycine uptake in the brain was evaluated using the brain uptake index method. Radiolabeled glycine was distributed among periventricular organs in the brain. Oral administration of 2 g/kg of glycine significantly elevated the CSF glycine concentration above the ED50 value for NMDA receptors. The glycine levels in CSF were 100 times lower than those in plasma. Glycine levels were elevated in brain tissue, but with a slower time-course than in CSF. Serine, a major metabolite of glycine, was elevated in plasma, CSF, and brain tissue. Glycine uptake in brain tissue increased in a dose-dependent manner. Time–concentration curves revealed that glycine was most likely transported via the blood–CSF barrier and activated NMDA receptors adjacent to the ventricles. The pharmacokinetic analysis and the brain uptake index for glycine suggested that glycine was transported into brain tissue by passive diffusion. These results provide further insight into the potential therapeutic applications of glycine.





Similar content being viewed by others
References
Bachmann C, Braissant O, Villard AM, Boulat O, Henry H (2004) Ammonia toxicity to the brain and creatine. Mol Genet Metab 81 Suppl 1:S52–S57. doi:10.1016/j.ymgme.2003.10.014
Bannai M, Kawai N, Nagao K, Nakano S, Matsuzawa D, Shimizu E (2011) Oral administration of glycine increases extracellular serotonin but not dopamine in the prefrontal cortex of rats. Psychiatry Clin Neurosci 65(2):142–149. doi:10.1111/j.1440-1819.2010.02181.x
Betz H, Gomeza J, Armsen W, Scholze P, Eulenburg V (2006) Glycine transporters: essential regulators of synaptic transmission. Biochem Soc Trans 34(Pt 1):55–58. doi:10.1042/BST0340055
Cape EG, Jones BE (2000) Effects of glutamate agonist versus procaine microinjections into the basal forebrain cholinergic cell area upon gamma and theta EEG activity and sleep–wake state. Eur J Neurosci 12(6):2166–2184. pii:ejn099
dos Santos Fagundes I, Rotta LN, Schweigert ID, Valle SC, de Oliveira KR, Huth Kruger A, Souza KB, Souza DO, Perr ML (2001) Glycine, serine, and leucine metabolism in different regions of rat central nervous system. Neurochem Res 26(3):245–249
D’Souza DC, Charney DS, Krystal JH (1995) Glycine site agonists of the NMDA receptor: a review. CNS Drug Rev 1(2):227–260. doi:10.1111/j.1527-3458.1995.tb00285.x
D’Souza DC, Gil R, Cassello K, Morrissey K, Abi-Saab D, White J, Sturwold R, Bennett A, Karper LP, Zuzarte E, Charney DS, Krystal JH (2000) IV glycine and oral d-cycloserine effects on plasma and CSF amino acids in healthy humans. Biol Psychiatry 47(5):450–462. pii:S0006-3223(99)00133-X
Gilmour G, Dix S, Fellini L, Gastambide F, Plath N, Steckler T, Talpos J, Tricklebank M (2011) NMDA receptors, cognition and schizophrenia—testing the validity of the NMDA receptor hypofunction hypothesis. Neuropharmacology. doi:10.1016/j.neuropharm.2011.03.015
Gomeza J, Hulsmann S, Ohno K, Eulenburg V, Szoke K, Richter D, Betz H (2003) Inactivation of the glycine transporter 1 gene discloses vital role of glial glycine uptake in glycinergic inhibition. Neuron 40(4):785–796
Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Silipo G, Lichtenstein M (1999) Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Arch Gen Psychiatry 56(1):29–36. doi:10.1001/archpsyc.56.1.29
Inagawa K, Hiraoka T, Kohda T, Yamadera W, Takahashi M (2006) Subjective effects of glycine ingestion before bedtime on sleep quality. Sleep and Biological Rhythms 4:75–77. doi:10.1111/j.1479-8425.2006.00193.x
Javitt DC, Zukin SR (1991) Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 148(10):1301–1308
Javitt DC, Zylberman I, Zukin SR, Heresco-Levy U, Lindenmayer JP (1994) Amelioration of negative symptoms in schizophrenia by glycine. Am J Psychiatry 151(8):1234–1236
Johnson JW, Ascher P (1987) Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 325(6104):529–531. doi:10.1038/325529a0
Juhasz G, Kekesi K, Emri Z, Soltesz I, Crunelli V (1990) Sleep-promoting action of excitatory amino acid antagonists: a different role for thalamic NMDA and non-NMDA receptors. Neurosci Lett 114(3):333–338
Lamers Y, Williamson J, Gilbert LR, Stacpoole PW, Gregory JF 3rd (2007) Glycine turnover and decarboxylation rate quantified in healthy men and women using primed, constant infusions of [1, 2-(13)C2]glycine and [(2)H3]leucine. J Nutr 137(12):2647–2652. pii:137/12/2647
Lin CH, Lane HY, Tsai GE (2011) Glutamate signaling in the pathophysiology and therapy of schizophrenia. Pharmacol Biochem Behav. doi:10.1016/j.pbb.2011.03.023
Milasius AM, Grinevicius KK, Lapin IP (1990) Effect of quinolinic acid on wakefulness and sleep in the rabbit. J Neural Transm Gen Sect 82(1):67–73
Moriki Y, Suzuki T, Fukami T, Hanano M, Tomono K, Watanabe J (2004) Involvement of P-glycoprotein in blood–brain barrier transport of pentazocine in rats using brain uptake index method. Biol Pharm Bull 27(6):932–935. doi:10.1248/bpb.27.932
Noguchi Y, Zhang QW, Sugimoto T, Furuhata Y, Sakai R, Mori M, Takahashi M, Kimura T (2006) Network analysis of plasma and tissue amino acids and the generation of an amino index for potential diagnostic use. Am J Clin Nutr 83(2):513S–519S. pii:83/2/513S
Oldendorf WH (1970) Measurement of brain uptake of radiolabeled substances using a tritiated water internal standard. Brain Res 24(2):372–376. pii:0006-8993(70)90123-X
Oldendorf WH (1971) Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am J Physiol 221(6):1629–1639
Pardridge WM, Oldendorf WH (1975) Kinetic analysis of blood–brain barrier transport of amino acids. Biochim Biophys Acta 401(1):128–136. pii:0005-2736(75)90347-8
Petzke KJ, Albrecht V, Przybilski H (1986) The influence of high glycine diets on the activity of glycine-catabolizing enzymes and on glycine catabolism in rats. J Nutr 116(5):742–750
Preston JE, Segal MB, Walley GJ, Zlokovic BV (1989) Neutral amino acid uptake by the isolated perfused sheep choroid plexus. J Physiol 408:31–43
Richter JJ, Wainer A (1971) Evidence for separate systems for the transport of neutral and basic amino acids across the blood–brain barrier. J Neurochem 18(4):613–620
Saunders NR, Habgood MD, Dziegielewska KM (1999) Barrier mechanisms in the brain, I. Adult brain. Clin Exp Pharmacol Physiol 26(1):11–19
Stone WS, Walker DL, Gold PE (1992) Sleep deficits in rats after NMDA receptor blockade. Physiol Behav 52(3):609–612. pii:0031-9384(92)90355-6
Stutzmann JM, Lucas M, Blanchard JC, Laduron PM (1988) Riluzole, a glutamate antagonist, enhances slow wave and REM sleep in rats. Neurosci Lett 88(2):195–200
Takahashi K, Hayashi F, Nishikawa T (1997) In vivo evidence for the link between L- and D-serine metabolism in rat cerebral cortex. J Neurochem 69(3):1286–1290
Toth E, Lajtha A (1981) Elevation of cerebral levels of nonessential amino acids in vivo by administration of large doses. Neurochem Res 6(12):1309–1317
Toth E, Lajtha A (1986) Antagonism of phencyclidine-induced hyperactivity by glycine in mice. Neurochem Res 11(3):393–400
Tsai G, Coyle JT (2002) Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol 42:165–179. doi:10.1146/annurev.pharmtox.42.082701.160735
Tsai G, Yang P, Chung LC, Lange N, Coyle JT (1998) d-serine added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry 44(11):1081–1089. pii:S0006-3223(98)00279-0
Tsai G, Lane HY, Yang P, Chong MY, Lange N (2004) Glycine transporter I inhibitor, N-methylglycine (sarcosine), added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry 55(5):452–456. doi:10.1016/j.biopsych.2003.09.012
Tuominen HJ, Tiihonen J, Wahlbeck K (2005) Glutamatergic drugs for schizophrenia: a systematic review and meta-analysis. Schizophr Res 72(2–3):225–234. doi:10.1016/j.schres.2004.05.005
Uchino H, Kanai Y, Kim DK, Wempe MF, Chairoungdua A, Morimoto E, Anders MW, Endou H (2002) Transport of amino acid-related compounds mediated by L-type amino acid transporter 1 (LAT1): insights into the mechanisms of substrate recognition. Mol Pharmacol 61(4):729–737
Verleysdonk S, Martin H, Willker W, Leibfritz D, Hamprecht B (1999) Rapid uptake and degradation of glycine by astroglial cells in culture: synthesis and release of serine and lactate. Glia 27(3):239–248. doi:10.1002/(SICI)1098-1136(199909)27:3<239:AID-GLIA5>3.0.CO;2-K
Wigren HK, Schepens M, Matto V, Stenberg D, Porkka-Heiskanen T (2007) Glutamatergic stimulation of the basal forebrain elevates extracellular adenosine and increases the subsequent sleep. Neuroscience 147(3):811–823. doi:10.1016/j.neuroscience.2007.04.046
Xue HH, Fujie M, Sakaguchi T, Oda T, Ogawa H, Kneer NM, Lardy HA, Ichiyama A (1999a) Flux of the l-serine metabolism in rat liver. The predominant contribution of serine dehydratase. J Biol Chem 274(23):16020–16027. doi:10.1074/jbc.274.23.16020
Xue HH, Sakaguchi T, Fujie M, Ogawa H, Ichiyama A (1999b) Flux of the l-serine metabolism in rabbit, human, and dog livers. Substantial contributions of both mitochondrial and peroxisomal serine:pyruvate/alanine:glyoxylate aminotransferase. J Biol Chem 274 (23):16028–16033. doi:10.1074/jbc.274.23.16028
Yamadera W, Inagawa K, Chiba S, Bannai M, Takahashi M, Namagawa K (2007) Glycine ingestion improves subjective sleep quality in human volunteers, correlating with polysomnographic changes. Sleep Biol Rhythms 5:126–131. doi:10.1111/j.1479-8425.2007.00262.x
Zafra F, Gimenez C (2008) Glycine transporters and synaptic function. IUBMB Life 60(12):810–817. doi:10.1002/iub.128
Acknowledgments
The authors express their gratitude to Drs. Y. Urade and Z. Huang from Osaka Bioscience Institute for their valuable input.
Conflict of interest
Nobuhiro Kawai, Makoto Bannai, Shinobu Seki, Kenji Nagao and Michio Takahashi are employees of Ajinomoto Co., Inc. Tomonori Koizumi and Kenji Shinkai are employees of Ajinomoto Pharmaceuticals Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawai, N., Bannai, M., Seki, S. et al. Pharmacokinetics and cerebral distribution of glycine administered to rats. Amino Acids 42, 2129–2137 (2012). https://doi.org/10.1007/s00726-011-0950-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-011-0950-y