Abstract
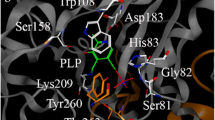
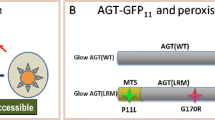
The G170R variant of the alanine:glyoxylate aminotransferase (AGT) is the most common pathogenic allele associated to primary hyperoxaluria type I (PH1), leading to mitochondrial mistargeting when combined with the P11L and I340M polymorphisms (minor allele; AGTLM). In this work, we have performed a comparative analysis on the conformation, unfolding energetics and interaction with molecular chaperones between AGTwt, AGTLM and AGTLRM (G170R in the minor allele) proteins. Our results show that these three variants share similar conformational and functional properties as folded dimers. However, kinetic stability analyses showed a ≈1,000-fold increased unfolding rate for apo-AGTLRM compared to apo-AGTwt, as well as a reduced folding efficiency upon expression in Escherichia coli. Pyridoxal 5′-phosphate (PLP)-binding provided a 4–5 orders of magnitude enhancement of the kinetic stability for all variants, suggesting a role for kinetic stabilization in pyridoxine-responsive PH1. Conformational studies at mild acidic pH and moderate guanidium concentrations showed the formation of a molten-globule-like unfolding intermediate in all three variants, which do not reactivate to the native state and strongly interact with Hsc70 and Hsp90 chaperones. Additional expression analyses in a mammalian cell-free system at neutral pH showed enhanced interaction of AGTLRM with Hsc70 and Hsp90 proteins compared to AGTwt, suggesting kinetic trapping of the mutant by chaperones along the folding process. Overall, our results suggest that mitochondrial mistargeting of AGTLRM may involve the presentation of AGT partially folded states to the mitochondrial import machinery by molecular chaperones, which would be facilitated by the low native state kinetic stability (partially corrected by PLP binding) and kinetic trapping during folding of the AGTLRM variant with molecular chaperones.







Similar content being viewed by others
Abbreviations
- AGT:
-
Alanine:glyoxylate aminotranferase
- ANS:
-
8-Anilinonaphthalene-1-sulfonic acid
- CD:
-
Circular dichroism
- DSC:
-
Differential scanning calorimetry
- PH1:
-
Primary hyperoxaluria type 1
- PLP:
-
Pyridoxal 5′-phosphate
- SEC:
-
Size-exclusion chromatography
References
Albert A, Yunta C, Arranz R, Pena A, Salido E, Valpuesta JM, Martin-Benito J (2010) Structure of GroEL in complex with an early folding intermediate of alanine glyoxylate aminotransferase. J Biol Chem 285:6371–6376
Allsop J, Jennings PR, Danpure CJ (1987) A new micro-assay for human liver alanine:glyoxylate aminotransferase. Clin Chim Acta 170:187–193
Artigues A, Iriarte A, Martinez-Carrion M (1994) Acid-induced reversible unfolding of mitochondrial aspartate aminotransferase. J Biol Chem 269:21990–21999
Artigues A, Iriarte A, Martinez-Carrion M (2002) Binding to chaperones allows import of a purified mitochondrial precursor into mitochondria. J Biol Chem 277:25047–25055
Baker MJ, Frazier AE, Gulbis JM, Ryan MT (2007) Mitochondrial protein-import machinery: correlating structure with function. Trends Cell Biol 17:456–464
Bohm G, Muhr R, Jaenicke R (1992) Quantitative analysis of protein far UV circular dichroism spectra by neural networks. Protein Eng 5:191–195
Brown LA, Baker A (2008) Shuttles and cycles: transport of proteins into the peroxisome matrix. Mol Membr Biol 25:363–375
Bukau B, Horwich AL (1998) The Hsp70 and Hsp60 chaperone machines. Cell 92:351–366
Bukau B, Weissman J, Horwich A (2006) Molecular chaperones and protein quality control. Cell 125:443–451
Campos LA, Sancho J (2003) The active site of pepsin is formed in the intermediate conformation dominant at mildly acidic pH. FEBS Lett 538:89–95
Cellini B, Bertoldi M, Montioli R, Paiardini A, Borri Voltattorni C (2007) Human wild-type alanine:glyoxylate aminotransferase and its naturally occurring G82E variant: functional properties and physiological implications. Biochem J 408:39–50
Cellini B, Montioli R, Bianconi S, Lopez-Alonso JP, Voltattorni CB (2008) Construction, purification and characterization of untagged human liver alanine-glyoxylate aminotransferase expressed in Escherichia coli. Protein Pept Lett 15:153–159
Cellini B, Montioli R, Paiardini A, Lorenzetto A, Voltattorni CB (2009) Molecular insight into the synergism between the minor allele of human liver peroxisomal alanine:glyoxylate aminotransferase and the F152I mutation. J Biol Chem 284:8349–8358
Cellini B, Montioli R, Paiardini A, Lorenzetto A, Maset F, Bellini T, Oppici E, Voltattorni CB (2010) Molecular defects of the glycine 41 variants of alanine glyoxylate aminotransferase associated with primary hyperoxaluria type I. Proc Natl Acad Sci USA 107:2896–2901
Coulter-Mackie MB, Lian Q, Wong SG (2005) Overexpression of human alanine:glyoxylate aminotransferase in Escherichia coli: renaturation from guanidine-HCl and affinity for pyridoxal phosphate co-factor. Protein Exp Purif 41:18–26
Cuellar J, Martin-Benito J, Scheres SH, Sousa R, Moro F, Lopez-Vinas E, Gomez-Puertas P, Muga A, Carrascosa JL, Valpuesta JM (2008) The structure of CCT-Hsc70 NBD suggests a mechanism for Hsp70 delivery of substrates to the chaperonin. Nat Struct Mol Biol 15:858–864
Danpure CJ (2006) Primary hyperoxaluria type 1: AGT mistargeting highlights the fundamental differences between the peroxisomal and mitochondrial protein import pathways. Biochim Biophys Acta 1763:1776–1784
Deu E, Kirsch JF (2007) Cofactor-directed reversible denaturation pathways: the cofactor-stabilized Escherichia coli aspartate aminotransferase homodimer unfolds through a pathway that differs from that of the apoenzyme. Biochemistry 46:5819–5829
Djordjevic S, Zhang X, Bartlam M, Ye S, Rao Z, Danpure CJ (2010) Structural implications of a G170R mutation of alanine:glyoxylate aminotransferase that is associated with peroxisome-to-mitochondrion mistargeting. Acta Crystallogr Sect F 66:233–236
Dobson CM (1994) Protein folding. Solid evidence for molten globules. Curr Biol 4:636–640
Dobson CM (2003) Protein folding and misfolding. Nature 426:884–890
Furth-Walker B, Leibman D, Smolen A (1990) Relationship between blood, liver and brain pyridoxal phosphate and pyridoxamine phosphate concentrations in mice. J Nutr 120:1338–1343
Griko YV, Privalov PL (1994) Thermodynamic puzzle of apomyoglobin unfolding. J Mol Biol 235:1318–1325
Halskau O, Muga A, Martinez A (2009) Linking new paradigms in protein chemistry to reversible membrane-protein interactions. Curr Protein Pept Sci 10:339–359
Hohfeld J, Cyr DM, Patterson C (2001) From the cradle to the grave: molecular chaperones that may choose between folding and degradation. EMBO Rep 2:885–890
Hopper ED, Pittman AM, Fitzgerald MC, Tucker CL (2008) In vivo and in vitro examination of stability of primary hyperoxaluria-associated human alanine:glyoxylate aminotransferase. J Biol Chem 283:30493–30502
Huber PA, Birdsey GM, Lumb MJ, Prowse DT, Perkins TJ, Knight DR, Danpure CJ (2005) Peroxisomal import of human alanine:glyoxylate aminotransferase requires ancillary targeting information remote from its C terminus. J Biol Chem 280:27111–27120
Kutik S, Guiard B, Meyer HE, Wiedemann N, Pfanner N (2007) Cooperation of translocase complexes in mitochondrial protein import. J Cell Biol 179:585–591
Lain B, Iriarte A, Martinez-Carrion M (1994) Dependence of the folding and import of the precursor to mitochondrial aspartate aminotransferase on the nature of the cell-free translation system. J Biol Chem 269:15588–15596
Liu Y, Cowan JA (2009) Iron-sulfur cluster biosynthesis: characterization of a molten globule domain in human NFU. Biochemistry 48:7512–7518
Lumb MJ, Danpure CJ (2000) Functional synergism between the most common polymorphism in human alanine:glyoxylate aminotransferase and four of the most common disease-causing mutations. J Biol Chem 275:36415–36422
Martinez A, Calvo AC, Teigen K, Pey AL (2008) Rescuing proteins of low kinetic stability by chaperones and natural ligands: phenylketonuria, a case study. Prog Mol Biol Transl Sci 83:89–134
Matouschek A, Fersht AR (1993) Application of physical organic chemistry to engineered mutants of proteins: Hammond postulate behavior in the transition state of protein folding. Proc Natl Acad Sci USA 90:7814–7818
Matouschek A, Otzen DE, Itzhaki LS, Jackson SE, Fersht AR (1995) Movement of the position of the transition state in protein folding. Biochemistry 34:13656–13662
Matouschek A, Azem A, Ratliff K, Glick BS, Schmid K, Schatz G (1997) Active unfolding of precursor proteins during mitochondrial protein import. EMBO J 16:6727–6736
Mattingly JR Jr, Iriarte A, Martinez-Carrion M (1995) Homologous proteins with different affinities for groEL. The refolding of the aspartate aminotransferase isozymes at varying temperatures. J Biol Chem 270:1138–1148
Meimaridou E, Gooljar SB, Chapple JP (2009) From hatching to dispatching: the multiple cellular roles of the Hsp70 molecular chaperone machinery. J Mol Endocrinol 42:1–9
Motley A, Lumb MJ, Oatey PB, Jennings PR, De Zoysa PA, Wanders RJ, Tabak HF, Danpure CJ (1995) Mammalian alanine/glyoxylate aminotransferase 1 is imported into peroxisomes via the PTS1 translocation pathway. Increased degeneracy and context specificity of the mammalian PTS1 motif and implications for the peroxisome-to-mitochondrion mistargeting of AGT in primary hyperoxaluria type 1. J Cell Biol 131:95–109
Noguchi T, Takada Y (1979) Peroxisomal localization of alanine:glyoxylate aminotransferase in human liver. Arch Biochem Biophys 196:645–647
Pace CN, Vajdos F, Fee L, Grimsley G, Gray T (1995) How to measure and predict the molar absorption coefficient of a protein. Protein Sci 4:2411–2423
Pey A, Saborido A, Blazquez I, Delgado J, Megias A (2003) Effect of prolonged stanozolol treatment on antioxidant enzyme activities, oxidative stress markers, and heat shock protein HSP72 levels in rat liver. J Steroid Biochem Mol Biol 87:269–277
Polverino de Laureto P, Vinante D, Scaramella E, Frare E, Fontana A (2001) Stepwise proteolytic removal of the beta subdomain in alpha-lactalbumin. The protein remains folded and can form the molten globule in acid solution. Eur J Biochem 268:4324–4333
Polverino de Laureto P, Frare E, Gottardo R, Van Dael H, Fontana A (2002) Partly folded states of members of the lysozyme/lactalbumin superfamily: a comparative study by circular dichroism spectroscopy and limited proteolysis. Protein Sci 11:2932–2946
Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE (2009) Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem 78:959–991
Prakash S, Matouschek A (2004) Protein unfolding in the cell. Trends Biochem Sci 29:593–600
Purdue PE, Allsop J, Isaya G, Rosenberg LE, Danpure CJ (1991) Mistargeting of peroxisomal l-alanine:glyoxylate aminotransferase to mitochondria in primary hyperoxaluria patients depends upon activation of a cryptic mitochondrial targeting sequence by a point mutation. Proc Natl Acad Sci USA 88:10900–10904
Robertson AD, Murphy KP (1997) Protein structure and the energetics of protein stability. Chem Rev 97:1251–1268
Rodriguez-Larrea D, Minning S, Borchert TV, Sanchez-Ruiz JM (2006) Role of solvation barriers in protein kinetic stability. J Mol Biol 360:715–724
Rumsby G, Weir T, Samuell CT (1997) A semiautomated alanine:glyoxylate aminotransferase assay for the tissue diagnosis of primary hyperoxaluria type 1. Ann Clin Biochem 34:400–404
Sanchez-Ruiz JM (1992) Theoretical analysis of Lumry-Eyring models in differential scanning calorimetry. Biophys J 61:921–935
Sanchez-Ruiz JM (2010) Protein kinetic stability. Biophys Chem 148:1–15
Sanchez-Ruiz JM, Lopez-Lacomba JL, Cortijo M, Mateo PL (1988) Differential scanning calorimetry of the irreversible thermal denaturation of thermolysin. Biochemistry 27:1648–1652
Santana A, Salido E, Torres A, Shapiro LJ (2003) Primary hyperoxaluria type 1 in the Canary Islands: a conformational disease due to I244T mutation in: the P11L-containing alanine:glyoxylate aminotransferase. Proc Natl Acad Sci USA 100:7277–7282
Wanders RJ, Ruiter J, Van Roermund CW, Schutgens RB, Ofman R, Jurriaans S, Tager JM (1990) Human liver l-alanine-glyoxylate aminotransferase: characteristics and activity in controls and hyperoxaluria type I patients using a simple spectrophotometric method. Clin Chim Acta 189:139–144
Wilcox AJ, Choy J, Bustamante C, Matouschek A (2005) Effect of protein structure on mitochondrial import. Proc Natl Acad Sci USA 43:15435–15440
Williams EL, Acquaviva C, Amoroso A, Chevalier F, Coulter-Mackie M, Monico CG, Giachino D, Owen T, Robbiano A, Salido E, Waterham H, Rumsby G (2009) Primary hyperoxaluria type 1: update and additional mutation analysis of the AGXT gene. Hum Mutat 30:910–917
Young JC, Hoogenraad NJ, Hartl FU (2003) Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell 112:41–50
Yutani K, Ogasahara K, Kuwajima K (1992) Absence of the thermal transition in apo-alpha-lactalbumin in the molten globule state. A study by differential scanning microcalorimetry. J Mol Biol 228:347–350
Zhang X, Roe SM, Hou Y, Bartlam M, Rao Z, Pearl LH, Danpure CJ (2003) Crystal structure of alanine:glyoxylate aminotransferase and the relationship between genotype and enzymatic phenotype in primary hyperoxaluria type 1. J Mol Biol 331:643–652
Acknowledgments
We want to thank Prof. Aurora Martinez at the University of Bergen for support at the initial stages of this work. This work was supported by grants from the Spanish Ministry of Education (SAF2007-62343 to E.S. and BIO2009–09562 and CSD2009-00088 to J.M.S-R). Angel L. Pey is supported by a Ramon y Cajal research contract from the Spanish Ministry of Science and Innovation (MICINN) and also acknowledges a short-term fellowship from the Federation of European Biochemical Societies (FEBS).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pey, A.L., Salido, E. & Sanchez-Ruiz, J.M. Role of low native state kinetic stability and interaction of partially unfolded states with molecular chaperones in the mitochondrial protein mistargeting associated with primary hyperoxaluria. Amino Acids 41, 1233–1245 (2011). https://doi.org/10.1007/s00726-010-0801-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-010-0801-2