Abstract
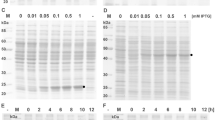
The human immunodeficiency virus type 1 (HIV-1) transactivator of transcription (TAT) protein, a member of the protein transduction domain (PTD) superfamily, can deliver heterologous proteins across most biomembranes without losing bioactivity. However, there is no report on whether the TAT core domain containing the sequence ‘YGRKKRRQRRR’ has other functions. As the TAT core domain is most basic (pI = 12.8) and has biomembrane crossing ability, we hypothesized it might probably influence the protein expression level due to subcellular redistribution of target proteins in the cells. To address this issue, we constructed the prokaryotic expression vector pET28b-TAT-EGFP (using the vector pET28b-EGFP for control) containing the core domain coding region, and transformed the vector into E. coli BL21 (DE3) cells for expression of the enhanced green fluorescent protein (EGFP) with the inducer isopropyl-β-d-thiogalactopyranoside (IPTG). Equal amount of the total proteins were fractionated using 15% SDS-PAGE and identified by western blot, and the plasmid copy number was assayed by Southern blot. In order to further study the subcellular localization of heterologous proteins in E. coli cells, the cytoplasmic and periplasmic components were extracted by chloroform and osmotic shock techniques. Interestingly, our data showed that the TAT core domain was not only able to promote the heterologous protein expression in E. coli, but also improve the yields and the solubility of heterologous proteins, while the plasmid copy number of TAT-containing clones and TAT-free clones was not affected by the TAT core domain. In addition, the TAT-tagged protein was mainly localized in the cytoplasm and also accumulated in the periplasmic space along with the time for protein expression, while in contrast, the TAT-free protein was mainly expressed in the periplasm and only a few in cytoplasm. A further examination on the distribution of the expressed proteins in cytoplasm and periplasm suggested that the TAT core domain might promote protein expression in the cytoplasm initially and then partially deliver them across the cytomembrane to the periplasmic space in a concentration-dependent manner. Taken together, our current data have provided a novel method for improving heterologous protein expression in prokaryotic cells by fusion with the TAT core domain, which will promote expression efficiency of bioactive proteins for protein engineering.







Similar content being viewed by others
References
Ames GF, Prody C et al (1984) Simple, rapid, and quantitative release of periplasmic proteins by chloroform. J Bacteriol 160(3):1181–1183
Baecker PA, Yung SG et al (1978) Periplasmic localization of nicotinate phosphoribosyltransferase in Escherichia coli. J Bacteriol 133(3):1108–1112
Beacham IR (1979) Periplasmic enzymes in gram-negative bacteria. Int J Biochem 10(11):877–883
Blackwell JR, Horgan R (1991) A novel strategy for production of a highly expressed recombinant protein in an active form. FEBS Lett 295(1–3):10–12
Burton N, Cavallini B et al (1991) Expression in Escherichia coli: purification and properties of the yeast general transcription factor TFIID. Protein Expr Purif 2(5–6):432–441
Caron NJ, Torrente Y et al (2001) Intracellular delivery of a TAT-EGFP fusion protein into muscle cells. Mol Ther 3(3):310–318
Derossi D, Joliot AH et al (1994) The third helix of the Antennapedia homeodomain translocates through biological membranes. J Biol Chem 269(14):10444–10450
Dubendorff JW, Studier FW (1991) Controlling basal expression in an inducible T7 expression system by blocking the target T7 promoter with lac repressor. J Mol Biol 219(1):45–59
Ewis HE, Lu CD (2005) Osmotic shock: a mechanosensitive channel blocker can prevent release of cytoplasmic but not periplasmic proteins. FEMS Microbiol Lett 253(2):295–301
Fawell S, Seery J et al (1994) Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci USA 91(2):664–668
Frankel AD, Pabo CO (1988) Cellular uptake of the tat protein from human immunodeficiency virus. Cell 55(6):1189–1193
Green M, Loewenstein PM (1988) Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 55(6):1179–1188
Hopper DJ, Jones MR et al (1985) Periplasmic location of p-cresol methyl hydroxylase in Pseudomonas putida. FEBS Lett 182(2):485–488
Jin LH, Bahn JH et al (2001) Transduction of human catalase mediated by an HIV-1 TAT protein basic domain and arginine-rich peptides into mammalian cells. Free Radic Biol Med 31(11):1509–1519
Joliot A, Pernelle C et al (1991) Antennapedia homeobox peptide regulates neural morphogenesis. Proc Natl Acad Sci USA 88(5):1864–1868
Kwon HY, Eum WS et al (2000) Transduction of Cu, Zn-superoxide dismutase mediated by an HIV-1 Tat protein basic domain into mammalian cells. FEBS Lett 485(2–3):163–167
Lewin M, Carlesso N et al (2000) Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol 18(4):410–414
Lindsay MA (2002) Peptide-mediated cell delivery: application in protein target validation. Curr Opin Pharmacol 2(5):587–594
Marvin HJ, Witholt B (1987) A highly efficient procedure for the quantitative formation of intact and viable lysozyme spheroplasts from Escherichia coli. Anal Biochem 164(2):320–330
Nagahara H, Vocero-Akbani AM et al (1998) Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat Med 4(12):1449–1452
Neu HC, Heppel LA (1964) The release of ribonuclease into the medium when E. coli cells are converted to spheroplasts. Biochem Biophys Res Commun 14:109–112
Nossal NG, Heppel LA (1966) The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem 241(13):3055–3062
Prochiantz A (2000) Messenger proteins: homeoproteins, TAT and others. Curr Opin Cell Biol 12(4):400–406
Ryu J, Han K et al (2003) Enhanced uptake of a heterologous protein with an HIV-1 Tat protein transduction domains (PTD) at both termini. Mol Cells 16(3):385–391
Ryu J, Lee HJ et al (2004) Intracellular delivery of p53 fused to the basic domain of HIV-1 Tat. Mol Cells 17(2):353–359
Schein CH (1991) Optimizing protein folding to the native state in bacteria. Curr Opin Biotechnol 2(5):746–750
Schwarze SR, Dowdy SF (2000) In vivo protein transduction: intracellular delivery of biologically active proteins, compounds and DNA. Trends Pharmacol Sci 21(2):45–48
Schwarze SR, Ho A et al (1999) In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 285(5433):1569–1572
Snyder EL, Dowdy SF (2004) Cell penetrating peptides in drug delivery. Pharm Res 21(3):389–393
Torchilin VP (2002) TAT peptide-modified liposomes for intracellular delivery of drugs and DNA. Cell Mol Biol Lett 7(2):265–267
Torchilin VP, Rammohan R et al (2001) TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc Natl Acad Sci USA 98(15):8786–8791
Vazquez-Laslop N, Lee H et al (2001) Molecular sieve mechanism of selective release of cytoplasmic proteins by osmotically shocked Escherichia coli. J Bacteriol 183(8):2399–2404
Vives E, Brodin P et al (1997) A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem 272(25):16010–16017
Vocero-Akbani AM, Heyden NV et al (1999) Killing HIV-infected cells by transduction with an HIV protease-activated caspase-3 protein. Nat Med 5(1):29–33
Wadia JS, Dowdy SF (2003) Modulation of cellular function by TAT mediated transduction of full length proteins. Curr Protein Pept Sci 4(2):97–104
Watson K, Edwards RJ (1999) HIV-1-trans-activating (Tat) protein: both a target and a tool in therapeutic approaches. Biochem Pharmacol 58(10):1521–1528
Wills KN, Atencio IA et al (2001) Intratumoral spread and increased efficacy of a p53-VP22 fusion protein expressed by a recombinant adenovirus. J Virol 75(18):8733–8741
Wu SP, Fu AL et al (2006) A novel therapeutic approach to 6-OHDA-induced Parkinson’s disease in rats via supplementation of PTD-conjugated tyrosine hydroxylase. Biochem Biophys Res Commun 346(1):1–6
Acknowledgments
The authors wish to thank the anonymous reviewers for their critical suggestions to improve the paper. This work is supported by The General Program (30973107, 30800196, 30772293) of the National Natural Science Foundation of China; The Special Key Programs for Science and Technology of China (2009ZX09301-002; 2009ZX09103-616; 2009ZX09503-002); The National Basic Research Project (973 program) (2006CB504100); The Major Program for Science and Technology Research of Beijing Municipal Bureau (7061004), and The Major Project for Tackling Key Problems on Science and Technology Research of Beijing Municipal Bureau (Z07000200540702).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, Y., Ren, C., Gao, Y. et al. A novel method for promoting heterologous protein expression in Escherichia coli by fusion with the HIV-1 TAT core domain. Amino Acids 39, 811–820 (2010). https://doi.org/10.1007/s00726-010-0534-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-010-0534-2