Abstract
Nosocomial infections often cause lethal pneumogenic sepsis. Information on early bacteria-host interaction in the lung is limited. In the present study, mice were sacrificed 60 min and 4 h after Pseudomonas aeruginosa (PA) infection to investigate lung morphology by using electron microscopy and light microscopy. After 1 h, bacteria were found in the alveoli partly in contact with surfactant. Alveolar macrophages were seen with up to 10 intracellular bacteria close to protrusions of alveolar epithelial type I cells and the gas/blood barrier. A rare but surprising finding was bacteria and even replicating bacteria in alveolar epithelial type II cells (AEII). No bacteria were seen in capillaries. Neither engulfment of bacteria by neutrophils nor structural damage of the pulmonary barrier was visible. After 4 h, many neutrophils were found within the capillaries, but also in the alveolar space. Thus, we hypothesize that, in early stages of infection, the uptake of PA even by single AEII can influence the course of the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nosocomial pneumonia is a severe problem in clinical medicine. It often causes pneumogenic sepsis and has a high mortality (Veesenmeyer et al. 2009). The gram-negative bacterium Pseudomonas aeruginosa (PA) is an opportunistic human pathogen causing chronic infections of the lung, for example, in cystic fibrosis. It is the most common bacillus in nosocomial infection of critically ill patients, particularly in intensive care units (Trautmann et al. 2005). PA infections vary from local to systemic and from acute to chronic infections. Nosocomial pneumonia in intensive care units is a significant medical problem responsible for high mortality and elevated costs in intubated and mechanically ventilated patients (Vonberg et al. 2008). Mortality rates reach 30%-60% for pneumonia, 27%-50% for bacteremia, and 80%-100% for bacteremic pneumonia. Therapeutic approaches are limited by the rapid multidrug resistance of PA. The most critical step is the translocation of bacteria from the air space into the circulation. This can be prevented by an intact pulmonary barrier of epithelial and endothelial cells and controlled by immune cells and surfactant (Herzog et al. 2008).
So far, the early interaction between PA and the pulmonary barrier has not been documented by using electron microscopy. We have investigated the initial bacteria-host interaction. Therefore, the time point of 60 min after inoculation has been chosen for electron-microscopic evaluation.
Materials and methods
PA (cystic fibrosis airway isolate TBCF10839) were grown in medium (LB) overnight at 37°C (230 rpm) to the stationary phase. The bacteria were centrifuged at 5000g for 10 min and washed twice with sterile phosphate-buffered saline (PBS). The optical density of the bacterial suspension was adjusted by spectrophotometry at 578 nm. The number of colony-forming units (CFU) was extrapolated from a standard growth curve, and appropriate dilutions with sterile PBS were made to prepare the inoculum for the mice. To verify the correct dilution, an aliquot was serially diluted on LB agar plates. Ten- to 12-week-old female BALB/c mice (Charles River, Sulzfeld, Germany) were inoculated with 30 μl of this bacterial suspension containing 1.0×108 CFU or 5×106 CFU via view-controlled intratracheal instillation as previously described (Herzog et al. 2008; Munder et al. 2002). At 1 h or 4 h after the infection, mice were killed by an overdose of normal anesthesia, and then the thorax was opened. Mice lungs were fixed by instillation (n=3) or perfusion (n=3) at each time point. Experiments were approved by the local governmental authorities and met the NIH Guidelines for the Care and Use of Laboratory Animals (NIH Publication No. 85. reprint 2002).
Fixation of lungs
A fixative consisting of 1.5% glutaraldehyde and 1.5% paraformaldehyde in 0.15 M HEPES buffer (with a total osmolarity of 800 mosmol/l and a vehicle osmolality of 300 mosm/kg at pH 7.35) was used.
Instillation fixation
A cannula was inserted into the trachea via tracheotomy. The chest was opened, and the heart-lung block was removed. The lungs were fixed by tracheal instillation at a constant pressure of 20 cm H2O in a special instillation device.
Perfusion fixation
After thoracotomy, the pericardial cavity was opened, and a cannula pushed forward into the pulmonary artery via the right ventricle. The cannula was then connected to a reservoir containing perfusion solutions. Lung perfusion was carried out at 20 cm H2O hydrostatic pressure. It was started with isotonic saline at room temperature to remove blood, and the left atrium was opened for volume relief. Immediately after 2-3 min pre-perfusion, the lung was inflated to prevent alveolar collapse, and perfusion fixation was carried out.
Tissue processing for electron microscopy
At the end of instillation or perfusion fixation, the trachea was tightened, and the heart-lung block was excised and immersed in the same fixative solution overnight at 4°C before further processing. After fixation, lungs were separated into right and left lungs, and each was cut into equidistant slices from apical to basal. The slices were taken for light and electron microscopy in an alternating manner. For electron microscopy, lung slices were cut into specimens sized 1 mm3. After repeated rinses in 0.1 mmol/l HEPES buffer and 0.1 mmol/l cacodylate buffer, samples were osmicated for 2 h in 1% osmium tetroxide in 0.1 mmol/l cacodylate buffer and rinsed again in 0.1 mmol/l cacodylate buffer repeatedly. After being rinsed twice in distilled water, specimens were stained en bloc overnight (12-18 h) in half-saturated aqueous uranyl acetate solution (1:1) at 4-8°C. Dehydration in an ascending series of acetone (70%, 90%, 100%) and embedding in Epon followed. Semi-thin (1 μm) and ultra-thin sections (70 nm) were cut by using an ultra-microtome (Reichert Ultracut S, Leica Microsystems, Wetzlar, Germany). Semi-thin sections were stained with toluidine-blue and with methylene-blue according to Loeffler (0.5 ml methylene-blue, 30 ml ethanol, 1 ml 1% potassium hydroxide solution, 99 ml twice-distilled water). Ultra-thin sections were stained with lead citrate and uranyl acetate. Qualitative investigations were carried out by using an electron microscope (EM 10, Zeiss, Oberkochen, Germany), and representative areas were documented. In addition, lung tissue was embedded in methacrylate, after which 2-μm-thick sections were stained according to Gram.
Results
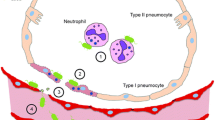
Mice were investigated 1 h after intratracheal infection with 1×108 CFU of the PA TBCF10839. Alveoli were found to be filled only sporadically with bacteria at this time point. The bacteria were seen preferentially in the hypophase of the alveoli close to active surfactant components (Fig. 1).
Bacteria in the alveolar space partly in contact with tubular myelin figures (tm), the active surfactant subtypes, 1 h after infection with colony-forming units of the PA strain (arrowheads). The alveolar space is bounded by alveolar epithelium type I (thin arrows). A capillary endothelial cell (E) with nucleus is also visible
Many bacteria had been engulfed by alveolar macrophages. A number of alveolar macrophages were found with up to 10 intracellular bacteria (Fig. 2). Unexpectedly, no neutrophils were seen with ingested PA.
Bacteria lying in the alveolar space (alv) and engulfed by alveolar macrophages at 1 h after infection with colony-forming units of the PA strain (thick arrows). No bacteria are visible within the capillaries (C) of the alveolar septa. Active (tubular myelin figures, small arrow) and inactive (uni-lamellar vesicles, arrow) subtypes of the intra-alveolar surfactant are seen near the bacteria and the alveolar macrophages
Surprisingly, alveolar epithelial type II cells (AEII) had taken up PA. The bacteria were observed to be replicating within the AEII, demonstrating the ongoing activity of the infection within these cells (Fig. 3). The frequency of this rare observation was clearly less than 1% (two AECII with bacteria and/or degradation products in 1000 AECII without visible bacteria).
Alveolar epithelial cell type II (AEII) with engulfed bacteria at 1 h after infection. Vacuoles with partially digested bacteria (arrowheads) lie in the epithelial cytoplasm. One vacuole contains a dividing bacterium (long arrow). The AEII is sitting on the basal lamina. Profiles of interstitial cells (IS) are seen in the interstitial space of the alveolar septum. Prolongations of alveolar epithelial cells (short arrows) and the alveolar space (alv) are also visible
In contrast, no AEI were found bearing intracellular PA. Cytoplasmic protrusions of alveolar macrophages containing PA-filled phagosomes were sometimes discovered close to the gas-blood barrier (Fig. 4, arrow). Even as early as 1 h after infection, nearly 50% of the observed alveolar macrophages contained bacteria. No PA were seen in capillaries or vessels (Fig. 4). Furthermore, no damage of the pulmonary barrier was detected (Fig. 4).
Abundant neutrophils were found at 4 h after the inoculation. No AECI or AECII were seen with intracellular bacteria. Again, no PA were seen within capillaries or vessels. Some of the intraalveolar neutrophils (15%) had also taken up bacteria.
PA were only poorly seen by light microscopy. A few PA could however be found in the lung parenchyma by light microscopy, as documented in preparations stained according to Gram (Figs. 5, 6).
Lung parenchyma 4 h after infection (Gram-staining of methacrylate semi-thin sections). a Numerous neutrophils (long arrow) and bacteria (short arrow, arrowhead) are present in some alveoli. However, alveoli without any bacteria and/or neutrophils are also visible. b Alveolar septa without bacteria in capillaries. Bacteria (short arrow) and an alveolar macrophage with intracellular bacteria (arrowheads, shown at higher magnification in the inset) are visible within the alveolar space
Lung parenchyma at 4 h after infection (methylene-blue staining of semi-thin sections; staining according to Loeffler). a Bacteria are clearly seen in the alveoli (arrowheads). AEII are also recognizable (arrows), because their lamellar bodies have in a different color when stained with methylene-blue (metachromasia). No bacteria are visible in capillaries. b Alveolar macrophages containing engulfed bacteria (arrows, insets) are visible in the alveolar space
Discussion
Pneumonia is the leading cause of sepsis and mortality in intensive care medicine (Herzog et al. 2008; Tsai and Grayson 2008). Gram-negative bacteria such as PA are the most common causes of death, especially in ventilator-associated pneumonia. Pulmonary defense extends from the structural barriers and multiple agents of the innate immune system up to cellular innate and adaptive immune responses. The course of infection is complex. Direct pathogen-induced host cell killing occurs (Hauser and Engel 1999; Rajan et al. 2000), but in parallel, the release or secretion of endo- and exotoxins induces a cytokine storm and a systemic inflammatory response (Marra et al. 2006). The host reaction and inflammation can lead to “collateral” damage, which itself is harmful (van der Poll and Opal 2008). The mechanisms of acute, subacute, and chronic infectious courses can vary with respect to bacteria-host interactions. Quorum sensing and the development of biofilms might be more important in chronic courses, e.g., during artificial ventilation for days and weeks (Kohler et al. 2009). The focus of this study has been to unravel morphologically the first steps of this cascade in an acute model of PA lung infection in mice, since this has not yet been described, to the best of our knowledge.
When mice were infected intratracheally with a supra-lethal inoculation dose, interestingly, only a few PA could be found in the lung parenchyma at 1 h after inoculation, as documented by using Gram stains (Fig. 5). This raised the question of immune evasion of the bacteria. The results showed intact structural barriers at 1 h after the beginning of the infection. An unexpected and rare finding was the infection of the AEII cells, which also contained replicating bacteria. Whether the bacteria were ingested by the AEII cells or had actively invaded them is unknown. The latter mechanism is more likely because of the effective secretion systems of the PA bacteria. Moreover, other workers have demonstrated the invasion of AEI and AEII cell cultures by bacteria (Keig et al. 2001; Zaas et al. 2005). This observation is interesting with respect to two main aspects. AEII are not “professional” phagocytes or defense cells and could enable the bacilli to escape from the alveolar “battleships”, viz., the alveolar macrophages. Furthermore, the infection of the AEII cells might impair surfactant homeostasis and the production of collectins as a result of the loss/death of AEII, or as a result of the functional disturbance of the surfactant secretion and reuptake machinery.
By 4 h after inoculation, the picture has changed. Abundant neutrophils are present. In contrast, bacteria can rarely be found. Infected AEII have not been observed. The results indicate an effective clearance by alveolar macrophages immediately after inoculation, followed by neutrophils within the next few hours. No bacteria are detected within the walls of vessels or capillaries at this stage, suggesting that the penetration of the lung blood barrier happens at later time points. However, if these are rare events, then they might have been missed.
The important role of AEII cells and their contribution to the immunological network in PA infection have been investigated during the last few years (Kannan et al. 2009). In the present study, we have demonstrated that AEII activates alveolar macrophage functions such as phagocytosis, a process that is mediated by the cytokine monocyte chemoattractant protein-1, which is released from AEII cells. The data indicate that AEII cells serve as an immune booster for fighting bacterial infections. Taken together, the results suggest that the overwhelming infection of the AEII results in a significant decrease of host defense in the alveoli. The measurement of surfactant function and the content of collectins should elucidate this problem.
PA elastase is known to degrade surfactant protein A (SP-A) and SP-D, which are both involved in antibacterial host defense (Mariencheck et al. 2003). In this context, the data from SP-A and SP-D knockout mice are important and clearly demonstrate the significant function of both collectins in PA infection (Giannoni et al. 2006).
References
Giannoni E, Sawa T, Allen L, Wiener-Kronish J, Hawgood S (2006) Surfactant proteins A and D enhance pulmonary clearance of Pseudomonas aeruginosa. Am J Respir Cell Mol Biol 34:704–710
Hauser AR, Engel JN (1999) Pseudomonas aeruginosa induces type-III-secretion-mediated apoptosis of macrophages and epithelial cells. Infect Immun 67:5530–5537
Herzog EL, Brody AR, Colby TV, Mason R, Williams MC (2008) Knowns and unknowns of the alveolus. Proc Am Thorac Soc 5:778–782
Kannan S, Huang H, Seeger D, Audet A, Chen Y, Huang C, Gao H, Li S, Wu M (2009) Alveolar epithelial type II cells activate alveolar macrophages and mitigate P. aeruginosa infection. PLoS ONE 4:e4891
Keig PM, Ingham E, Kerr KG (2001) Invasion of human type II pneumocytes by Burkholderia cepacia. Microb Pathog 30:167–170
Kohler T, Buckling A, Delden C van (2009) Cooperation and virulence of clinical Pseudomonas aeruginosa populations. Proc Natl Acad Sci USA 106:6339–6344
Mariencheck WI, Alcorn JF, Palmer SM, Wright JR (2003) Pseudomonas aeruginosa elastase degrades surfactant proteins A and D. Am J Respir Cell Mol Biol 28:528–537
Marra AR, Bar K, Bearman GM, Wenzel RP, Edmond MB (2006) Systemic inflammatory response syndrome in adult patients with nosocomial bloodstream infection due to Pseudomonas aeruginosa. J Infect 53:30–35
Munder A, Krusch S, Tschernig T, Dorsch M, Luhrmann A, Griensven M van, Tummler B, Weiss S, Hedrich HJ (2002) Pulmonary microbial infection in mice: comparison of different application methods and correlation of bacterial numbers and histopathology. Exp Toxicol Pathol 54:127–133
Rajan S, Cacalano G, Bryan R, Ratner AJ, Sontich CU, Heerckeren A van, Davis P, Prince A (2000) Pseudomonas aeruginosa induction of apoptosis in respiratory epithelial cells: analysis of the effects of cystic fibrosis transmembrane conductance regulator dysfunction and bacterial virulence factors. Am J Respir Cell Mol Biol 23:304–312
Trautmann M, Lepper PM, Haller M (2005) Ecology of Pseudomonas aeruginosa in the intensive care unit and the evolving role of water outlets as a reservoir of the organism. Am J Infect Control 33:S41–S49
Tsai KS, Grayson MH (2008) Pulmonary defense mechanisms against pneumonia and sepsis. Curr Opin Pulm Med 14:260–265
van der Poll T, Opal SM (2008) Host-pathogen interactions in sepsis. Lancet Infect Dis 8:32–43
Veesenmeyer JL, Hauser AR, Lisboa T, Rello J (2009) Pseudomonas aeruginosa virulence and therapy: evolving translational strategies. Crit Care Med 37:1777–1786
Vonberg RP, Wolter A, Chaberny IF, Kola A, Ziesing S, Suerbaum S, Gastmeier P (2008) Epidemiology of multi-drug-resistant gram-negative bacteria: data from an university hospital over a 36-month period. Int J Hyg Environ Health 211:251–257
Zaas DW, Duncan MJ, Li G, Wright JR, Abraham SN (2005) Pseudomonas invasion of type I pneumocytes is dependent on the expression and phosphorylation of caveolin-2. J Biol Chem 280:4864–4872
Acknowledgements
The authors are grateful to D. Leemhuis and S. Fassbender for excellent technical assistance and to S. Fryk for correction of the language. For discussions and advice, the authors thank experts in the field: Matthias Ochs (Hannover/Germany), Helmut Bartels (Munich/Germany), and Pedro Mestres Ventura (Madrid/Spain).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmiedl, A., Kerber-Momot, T., Munder, A. et al. Bacterial distribution in lung parenchyma early after pulmonary infection with Pseudomonas aeruginosa . Cell Tissue Res 342, 67–73 (2010). https://doi.org/10.1007/s00441-010-1036-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-010-1036-y