Abstract
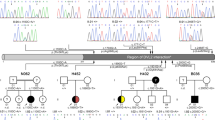
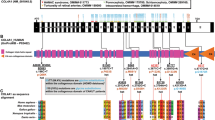
Congenital anomalies of the kidneys and urinary tract (CAKUT) are genetically highly heterogeneous leaving most cases unclear after mutational analysis of the around 30 causative genes known so far. Assuming that phenotypes frequently showing dominant inheritance, such as CAKUT, can be caused by de novo mutations, de novo analysis of whole-exome sequencing data was done on two patient–parent-trios to identify novel CAKUT genes. In one case, we detected a heterozygous de novo frameshift variant in TBC1D1 encoding a Rab-GTPase-activating protein regulating glucose transporter GLUT4 translocation. Sequence analysis of 100 further CAKUT cases yielded three novel or rare inherited heterozygous TBC1D1 missense variants predicted to be pathogenic. TBC1D1 mutations affected Ser237-phosphorylation or protein stability and thereby act as hypomorphs. Tbc1d1 showed widespread expression in the developing murine urogenital system. A mild CAKUT spectrum phenotype, including anomalies observed in patients carrying TBC1D1 mutations, was found in kidneys of some Tbc1d1 −/− mice. Significantly reduced Glut4 levels were detected in kidneys of Tbc1d1 −/− mice and the dysplastic kidney of a TBC1D1 mutation carrier versus controls. TBC1D1 and SLC2A4 encoding GLUT4 were highly expressed in human fetal kidney. The patient with the truncating TBC1D1 mutation showed evidence for insulin resistance. These data demonstrate heterozygous deactivating TBC1D1 mutations in CAKUT patients with a similar renal and ureteral phenotype, and provide evidence that TBC1D1 mutations may contribute to CAKUT pathogenesis, possibly via a role in glucose homeostasis.






Similar content being viewed by others
References
An D, Toyoda T, Taylor EB, Yu H, Fujii N, Hirshman MF, Goodyear LJ (2010) TBC1D1 regulates insulin- and contraction-induced glucose transport in mouse skeletal muscle. Diabetes 59:1358–1365. doi:10.2337/db09-1266
Boycott KM, Vanstone MR, Bulman DE, MacKenzie AE (2013) Rare-disease genetics in the era of next-generation sequencing: discovery to translation. Nat Rev Genet 14:681–691. doi:10.1038/nrg3555
Chadt A, Leicht K, Deshmukh A, Jiang LQ, Scherneck S, Bernhardt U, Dreja T, Vogel H, Schmolz K, Kluge R, Zierath JR, Hultschig C, Hoeben RC, Schürmann A, Joost HG, Al-Hasani H (2008) Tbc1d1 mutation in lean mouse strain confers leanness and protects from diet-induced obesity. Nat Genet 40:1354–1359. doi:10.1038/ng.244
Chadt A, Immisch A, de Wendt C, Springer C, Zhou Z, Stermann T, Holman GD, Loffing-Cueni D, Loffing J, Joost HG, Al-Hasani H (2015) Deletion of both Rab-GTPase-activating proteins TBC1D1 and TBC1D4 in mice eliminates insulin- and AICAR-stimulated glucose transport. Diabetes 64:746–759. doi:10.2337/db15-er04
Chen S, Murphy J, Toth R, Campbell DG, Morrice NA, Mackintosh C (2008) Complementary regulation of TBC1D1 and AS160 by growth factors, insulin and AMPK activators. Biochem J 409:449–459
Classen CF, Riehmer V, Landwehr C, Kosfeld A, Heilmann S, Scholz C, Kabisch S, Engels H, Tierling S, Zivicnjak M, Schacherer F, Haffner D, Weber RG (2013) Dissecting the genotype in syndromic intellectual disability using whole exome sequencing in addition to genome-wide copy number analysis. Hum Genet 132:825–841. doi:10.1007/s00439-013-1296-1
Cooper DN, Krawczak M, Polychronakos C, Tyler-Smith C, Kehrer-Sawatzki H (2013) Where genotype is not predictive of phenotype: towards an understanding of the molecular basis of reduced penetrance in human inherited disease. Hum Genet 132:1077–1130. doi:10.1007/s00439-013-1331-2
Dash S, Sano H, Rochford JJ, Semple RK, Yeo G, Hyden CS, Soos MA, Clark J, Rodin A, Langenberg C, Druet C, Fawcett KA, Tung YC, Wareham NJ, Barroso I, Lienhard GE, O’Rahilly S, Savage DB (2009) A truncation mutation in TBC1D4 in a family with acanthosis nigricans and postprandial hyperinsulinemia. Proc Natl Acad Sci 106:9350–9355. doi:10.1073/pnas.0900909106
Davis TK, Hoshi M, Jain S (2014) To bud or not to bud: the RET perspective in CAKUT. Pediatr Nephrol 29:597–608
de Ligt J, Willemsen MH, van Bon BW, Kleefstra T, Yntema HG, Kroes T, Vulto-van Silfhout AT, Koolen DA, de Vries P, Gilissen C, del Rosario M, Hoischen A, Scheffer H, de Vries BB, Brunner HG, Veltman JA, Vissers LE (2012) Diagnostic exome sequencing in persons with severe intellectual disability. N Engl J Med 367:1921–1929. doi:10.1056/NEJMoa1206524
Dokas J, Chadt A, Nolden T, Himmelbauer H, Zierath JR, Joost HG, Al-Hasani H (2013) Conventional knockout of Tbc1d1 in mice impairs insulin- and AICAR-stimulated glucose uptake in skeletal muscle. Endocrinology 154:3502–3514. doi:10.1210/en.2012-2147
Dorschner MO, Amendola LM, Turner EH, Robertson PD, Shirts BH, Gallego CJ, Bennett RL, Jones KL, Tokita MJ, Bennett JT, Kim JH, Rosenthal EA, Kim DS, National Heart, Lung, and Blood Institute Grand Opportunity Exome Sequencing Project, Tabor HK, Bamshad MJ, Motulsky AG, Scott CR, Pritchard CC, Walsh T, Burke W, Raskind WH, Byers P, Hisama FM, Nickerson DA, Jarvik GP (2013) Actionable, pathogenic incidental findings in 1,000 participants’ exomes. Am J Hum Genet 93:631–640. doi:10.1016/j.ajhg.2013.08.006
El Nahas AM, Bassett AH, Cope GH, Le Carpentier JE (1991) Role of growth hormone in the development of experimental renal scarring. Kidney Int 40:29–34
Fontanesi L, Bertolini F (2013) The TBC1D1 gene: structure, function, and association with obesity and related traits. Vitam Horm 91:77–95. doi:10.1016/B978-0-12-407766-9.00004-3
Frøsig C, Pehmøller C, Birk JB, Richter EA, Wojtaszewski JF (2010) Exercise-induced TBC1D1 Ser237 phosphorylation and 14-3-3 protein binding capacity in human skeletal muscle. J Physiol 588:4539–4548. doi:10.1113/jphysiol.2010.194811
Goumenos DS, Brown CB, Shortland J, el Nahas AM (1994) Myofibroblasts, predictors of progression of mesangial IgA nephropathy? Nephrol Dial Transplant 9:1418–1425
Guzman J, Jauregui AN, Merscher-Gomez S, Maiguel D, Muresan C, Mitrofanova A, Diez-Sampedro A, Szust J, Yoo TH, Villarreal R, Pedigo C, Molano RD, Johnson K, Kahn B, Hartleben B, Huber TB, Saha J, Burke GW 3rd, Abel ED, Brosius FC, Fornoni A (2014) Podocyte-specific GLUT4-deficient mice have fewer and larger podocytes and are protected from diabetic nephropathy. Diabetes 63:701–714. doi:10.2337/db13-0752
Haas CS, Amann K, Schittny J, Blaser B, Müller U, Hartner A (2003) Glomerular and renal vascular structural changes in alpha8 integrin-deficient mice. J Am Soc Nephrol 14:2288–2296
Harambat J, van Stralen KJ, Kim JJ, Tizard EJ (2012) Epidemiology of chronic kidney disease in children. Pediatr Nephrol 27:363–373. doi:10.1007/s00467-011-1939-1
Humbert C, Silbermann F, Morar B, Parisot M, Zarhrate M, Masson C, Tores F, Blanchet P, Perez MJ, Petrov Y, Khau Van Kien P, Roume J, Leroy B, Gribouval O, Kalaydjieva L, Heidet L, Salomon R, Antignac C, Benmerah A, Saunier S, Jeanpierre C (2014) Integrin alpha 8 recessive mutations are responsible for bilateral renal agenesis in humans. Am J Hum Genet 94:288–294. doi:10.1016/j.ajhg.2013.12.017
Hwang DY, Dworschak GC, Kohl S, Saisawat P, Vivante A, Hilger AC, Reutter HM, Soliman NA, Bogdanovic R, Kehinde EO, Tasic V, Hildebrandt F (2014) Mutations in 12 known dominant disease-causing genes clarify many congenital anomalies of the kidney and urinary tract. Kidney Int 85:1429–1433. doi:10.1038/ki.2013.508
Hwang DY, Kohl S, Fan X, Vivante A, Chan S, Dworschak GC, Schulz J, van Eerde AM, Hilger AC, Gee HY, Pennimpede T, Herrmann BG, van de Hoek G, Renkema KY, Schell C, Huber TB, Reutter HM, Soliman NA, Stajic N, Bogdanovic R, Kehinde EO, Lifton RP, Tasic V, Lu W, Hildebrandt F (2015) Mutations of the SLIT2-ROBO2 pathway genes SLIT2 and SRGAP1 confer risk for congenital anomalies of the kidney and urinary tract. Hum Genet 134:905–916. doi:10.1007/s00439-015-1570-5
Ichikawa I, Kuwayama F, Pope JC 4th, Stephens FD, Miyazaki Y (2002) Paradigm shift from classic anatomic theories to contemporary cell biological views of CAKUT. Kidney Int 61:889–898
Jeanpierre C, Macé G, Parisot M, Morinière V, Pawtowsky A, Benabou M, Martinovic J, Amiel J, Attié-Bitach T, Delezoide AL, Loget P, Blanchet P, Gaillard D, Gonzales M, Carpentier W, Nitschke P, Tores F, Heidet L, Antignac C, Salomon R, Société Française de Foetopathologie (2011) RET and GDNF mutations are rare in fetuses with renal agenesis or other severe kidney development defects. J Med Genet 48:497–504. doi:10.1136/jmg.2010.088526
Johnston JJ, Rubinstein WS, Facio FM, Ng D, Singh LN, Teer JK, Mullikin JC, Biesecker LG (2012) Secondary variants in individuals undergoing exome sequencing: screening of 572 individuals identifies high-penetrance mutations in cancer-susceptibility genes. Am J Hum Genet 91:97–108. doi:10.1016/j.ajhg.2012.05.021
Kane S, Sano H, Liu SC, Asara JM, Lane WS, Garner CC, Lienhard GE (2002) A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem 277:22115–22118
Katz EB, Stenbit AE, Hatton K, DePinho R, Charron MJ (1995) Cardiac and adipose tissue abnormalities but not diabetes in mice deficient in GLUT4. Nature 377:151–155
Kjær S, Hanrahan S, Totty N, McDonald NQ (2010) Mammal-restricted elements predispose human RET to folding impairment by HSCR mutations. Nat Struct Mol Biol 17:726–731. doi:10.1038/nsmb.1808
Limwongse C (2009) Syndromes and malformations of the urinary tract. In: Avner ED, Harman WE, Niaudet P, Yoshikawa N (eds) Pediatric Nephrology, 6th edn. Springer, Berlin, pp 121–156
Moorman AF, Houweling AC, de Boer PA, Christoffels VM (2001) Sensitive nonradioactive detection of mRNA in tissue sections: novel application of the whole-mount in situ hybridization protocol. J Histochem Cytochem 49:1–8
Pan X, Eathiraj S, Munson M, Lambright DG (2006) TBC-domain GAPs for Rab GTPases accelerate GTP hydrolysis by a dual-finger mechanism. Nature 442:303–306
Roach WG, Chavez JA, Mîinea CP, Lienhard GE (2007) Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1. Biochem J 403:353–358
Saisawat P, Kohl S, Hilger AC, Hwang DY, Yung Gee H, Dworschak GC, Tasic V, Pennimpede T, Natarajan S, Sperry E, Matassa DS, Stajić N, Bogdanovic R, de Blaauw I, Marcelis CL, Wijers CH, Bartels E, Schmiedeke E, Schmidt D, Märzheuser S, Grasshoff-Derr S, Holland-Cunz S, Ludwig M, Nöthen MM, Draaken M, Brosens E, Heij H, Tibboel D, Herrmann BG, Solomon BD, de Klein A, van Rooij IA, Esposito F, Reutter HM, Hildebrandt F (2014) Whole-exome resequencing reveals recessive mutations in TRAP1 in individuals with CAKUT and VACTERL association. Kidney Int 85:1310–1317. doi:10.1038/ki.2013.417
Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE (2003) Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem 278:14599–14602
Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F, Pachnis V (1994) Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367:380–383
Steinberg KM, Yu B, Koboldt DC, Mardis ER, Pamphlett R (2015) Exome sequencing of case-unaffected-parents trios reveals recessive and de novo genetic variants in sporadic ALS. Sci Rep 5:9124. doi:10.1038/srep09124
Stenbit AE, Tsao TS, Li J, Burcelin R, Geenen DL, Factor SM, Houseknecht K, Katz EB, Charron MJ (1997) GLUT4 heterozygous knockout mice develop muscle insulin resistance and diabetes. Nat Med 3:1096–1101
Vivante A, Kohl S, Hwang DY, Dworschak GC, Hildebrandt F (2014) Single-gene causes of congenital anomalies of the kidney and urinary tract (CAKUT) in humans. Pediatr Nephrol 29:695–704. doi:10.1007/s00467-013-2684-4
Weibel ER (1979) Morphometry of the human lung: the state of the art after two decades. Bull Eur Physiopathol Respir 15:999–1013
Wilkinson DG, Nieto MA (1993) Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol 225:361–373
Yosypiv IV (2012) Congenital anomalies of the kidney and urinary tract: a genetic disorder? Int J Nephrol 2012:909083. doi:10.1155/2012/909083
Acknowledgments
The authors wish to thank the patients and their families for participating in this study. This work was supported by the Else Kröner-Fresenius-Stiftung (Grant no. 2014_A234).
Author information
Authors and Affiliations
Corresponding author
Additional information
A. Kosfeld and M. Kreuzer contributed equally as first authors and D. Haffner and R. G. Weber contributed equally as senior authors.
Rights and permissions
About this article
Cite this article
Kosfeld, A., Kreuzer, M., Daniel, C. et al. Whole-exome sequencing identifies mutations of TBC1D1 encoding a Rab-GTPase-activating protein in patients with congenital anomalies of the kidneys and urinary tract (CAKUT). Hum Genet 135, 69–87 (2016). https://doi.org/10.1007/s00439-015-1610-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-015-1610-1