Abstract
Development of protective immunity against Plasmodium falciparum is partially mediated through binding of malaria-specific IgG to Fc gamma (γ) receptors. Variations in human FcγRIIA-H/R-131 and FcγRIIIB-NA1/NA2 affect differential binding of IgG sub-classes. Since variability in FcγR may play an important role in severe malarial anemia (SMA) pathogenesis by mediating phagocytosis of red blood cells and triggering cytokine production, the relationship between FcγRIIA-H/R131 and FcγRIIIB-NA1/NA2 haplotypes and susceptibility to SMA (Hb < 6.0 g/dL) was investigated in Kenyan children (n = 528) with acute malaria residing in a holoendemic P. falciparum transmission region. In addition, the association between carriage of the haplotypes and repeated episodes of SMA and all-cause mortality were investigated over a 3-year follow-up period. Since variability in FcγR can alter interferon (IFN)-γ production, a mediator of innate and adaptive immune responses, functional associations between the haplotypes and IFN-γ were also explored. During acute malaria, children with SMA had elevated peripheral IFN-γ levels (P = 0.006). Although multivariate logistic regression analyses (controlling for covariates) revealed no associations between the FcγR haplotypes and susceptibility to SMA during acute infection, the FcγRIIA-131H/FcγRIIIB-NA1 haplotype was associated with decreased peripheral IFN-γ (P = 0.046). Longitudinal analyses showed that carriage of the FcγRIIA-131H/FcγRIIIB-NA1 haplotype was associated with reduced risk of SMA (RR 0.65, 95% CI 0.46–0.90; P = 0.012) and all-cause mortality (P = 0.002). In contrast, carriers of the FcγRIIA-131H/FcγRIIIB-NA2 haplotype had increased susceptibility to SMA (RR 1.47, 95% CI 1.06–2.04; P = 0.020). Results here demonstrate that variation in the FcγR gene alters susceptibility to repeated episodes of SMA and mortality, as well as functional changes in IFN-γ production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plasmodium falciparum malaria remains one of the most common causes of morbidity and mortality in African children with severe manifestations of disease defined by the following clinical sequeleae: high-density parasitemia (HDP ≥10,000 parasites/μL); hypoglycemia; severe malarial anemia [SMA; hemoglobin (Hb) <6.0 g/dL]; cerebral malaria (CM); and respiratory distress (Bloland et al. 1999b; English et al. 1998; Mockenhaupt et al. 2004; Ong’echa et al. 2006). Naturally acquired antibodies are important for protection against asexual blood stages of malaria as evidenced by passive transfer of immunoglobulin G (IgG) from malaria-immune adults to malaria-naive children (Sabchareon et al. 1991). Binding of the heavy chain immunoglobulin domain to Fc receptors on phagocytic cells is important for protective immunity against malaria (Sabchareon et al. 1991). Fc gamma receptors IIA (FcγRIIA) and FcγRIIIB are low-affinity Fcγ-receptors expressed on monocytes/macrophages, and other immune cells, that interact with complex (or aggregated) IgG subtypes (IgG1–4) (Stein et al. 2000), thereby providing an important link between cellular and humoral malarial immunity.
FcγRIIA has co-dominantly expressed allotypes, differing by one amino acid (histidine or arginine) at position 131 (FcγRIIA-131H/R). A previous study in a holoendemic P. falciparum transmission area of western Kenya illustrated that the R/R131 genotype, that preferentially binds IgG1 and IgG3 (Bouharoun-Tayoun et al. 1995), was associated with protection against intermediate levels of parasitemia (i.e., >5,000 parasites/μL) relative to the H/R131 genotype (Shi et al. 2001). In addition, our previous investigation in an adjacent region demonstrated that the R/R131 genotype was significantly associated with protection against HDP (≥10,000 parasites/μL) (Ouma et al. 2006). Studies in The Gambia also showed that homozygous H131 alleles, with preferential binding to IgG2 (Warmerdam et al. 1991), increased susceptibility to malaria in children with a mixed clinical phenotype of severe disease characterized by CM, SMA, and/or hypoglycemia (Cooke et al. 2003).
FcγRIIIB is constitutively expressed on neutrophils with two of the allelic forms (NA1 and NA2) differing at four amino acid positions, including two potential glycosylation sites. Expression of FcγRIIIB-NA1/NA2 influences the phagocytic capacity of neutrophils as evidenced by studies showing that the FcγRIIIB-NA2/NA2 genotype was associated with reduced phagocytosis relative to homozygous FcγRIIIB-NA1 (Salmon et al. 1990). Haplotypic studies in Thai adults further revealed that co-carriage of the FcγRIIIB-NA2 and FcγRIIA-HH131 alleles increased susceptibility to CM (Omi et al. 2002). However, the association between these haplotypes and susceptibility to severe malaria in holoendemic P. falciparum transmission areas has not been previously reported.
Interferon-gamma (IFN-γ) is a multifunctional cytokine produced primarily from T lymphocytes and natural killer (NK)-cells, which plays an important role in modulating the immune response (Miller et al. 2009). Based on the fact that IFN-γ is associated with development of a type 1 (cell-mediated) immune response, this cytokine forms an integral part of innate immunity (Gajewski et al. 1989; Chehimi and Trinchieri 1994; Kobayashi et al. 2000). A previous study showed that higher malaria-specific production of IFN-γ and elevated anti-malarial IgG and IgE were associated with protection against malaria in West African populations (Farouk et al. 2005). Recent investigations in Thai adults also demonstrated significant differences in IFN-γ levels between patients with complicated and uncomplicated malaria (Tangteerawatana et al. 2007).
Since elevated IFN-γ production is associated with reduced levels of P. falciparum parasitemia (Torre et al. 2002b), along with data showing that IFN-γ bridges innate and adaptive immunity through engagement of immunoglobulins (Janeway and Medzhitov 2002), we reasoned that variability in FcγRIIA and FcγRIIIB may condition susceptibility to severe malaria by altering the production of IFN-γ. Our previous investigations in the cohort investigated here demonstrated that multi-site haplotypes are highly informative allelic markers that can reveal associations with malaria disease outcomes not identifiable with single polymorphisms (Ouma et al. 2008a, b). As such, the role of FcγRIIA-131H/R and FcγRIIIB-NA1/NA2 haplotypes in mediating susceptibility to SMA and functional changes in IFN-γ levels were examined in children with acute malaria (n = 528; 3–36 months) in a holoendemic P. falciparum transmission area of western Kenya. In addition, the relationship between the haplotypes, repeated episodes of SMA, and all-cause mortality were also investigated and we identified children (n = 528), who had complete data on malaria morbidity over a 3-year follow-up period, and included these in longitudinal analyses.
Methods
Study participants
Children with P. falciparum malaria were recruited at Siaya District Hospital (SDH), western Kenya. A total of 528 study participants were included in the current investigation for both the cross-sectional (acute malaria) and longitudinal analyses. In this holoendemic P. falciparum transmission area (Bloland et al. 1999b), SMA and hyperparasitemia are the most common clinical manifestations of severe malaria, with CM occurring only in rare cases (Bloland et al. 1999a; Ong’echa et al. 2006). Greater than 99% of the study participants were from the Luo ethnic group. Children with malaria were stratified into non-severe malarial anemia (non-SMA; Hb ≥ 6.0 g/dL) and severe malarial anemia (SMA; Hb < 6.0 g/dL) groups (McElroy et al. 1999). Administration of antimalarials and appropriate supportive therapy was provided for all children as per Kenya Ministry of Health guidelines. None of the children included in the study had non-falciparum parasitemia and/or CM. Written informed consent in the language of choice (i.e., English, Kiswahili, or Dholuo) was obtained from the parents/guardians of participating children. The study was approved by the Institutional Review Boards at the University of Pittsburgh and University of New Mexico, and the Kenya Medical Research Institute Ethical Review Board.
Laboratory procedures
Venous blood samples (<3.0 mL) were obtained prior to the administration of antimalarials and/or any other treatment interventions. Asexual malaria parasites (trophozoites) were counted against 300 leukocytes in peripheral blood smears stained with 3% Giemsa. Parasite density was estimated using the white blood cell (WBC) count/μL of each individual. Complete hematological parameters were determined with a Beckman Coulter® AcT diff2™ (Beckman-Coulter Corporation). Presences of the sickle cell trait (HbAS), and Glucose-6-Phosphate Dehydrogenase (G6PD) deficiency, were determined as previously described (Ouma et al. 2010). HIV-1 exposure and infection were determined by serological (i.e., Unigold™ and Determine™) and HIV-1 proviral DNA PCR testing, respectively, according to our previously published methods (Otieno et al. 2006). Pre- and post-test HIV counseling was provided for all participants. Trimethoprim–sulfamethoxazole was administered to all children that were positive for one or both serological HIV-1 tests. At the time of the sample collection, none of the HIV-1(+) study participants had been initiated on antiretroviral treatment. Bacteremia was determined using Wampole™ Isostat® Pediatric 1.5 system (Inverness Medical) and blood was processed according to the manufacturer’s instructions. API biochemical galleries (bioMerieux, Inc.) and/or serology were used for the identification of bacterial isolates as previously described (Were et al. 2011).
Genotyping
DNA was extracted from blood spotted on FTA® Classic cards (Whatman Inc.) using the Chelex method (Vignoli et al. 1995). The genomic DNA was then amplified using a DNA Amplification Kit via a strand displacement reaction (GE Healthcare). FcγRIIA-131H/R genotypes were determined using previously described methods by gene-specific PCR amplification on a PTC-100™ thermocycler (MJ Research, Inc.) followed by allele-specific restriction enzyme digestion with BstUI (New England Biolabs) (Jiang et al. 1996). BstUI digestion of the PCR product (366 bp) produced a 322-bp fragment for the RR131 genotype, a 343-bp fragment for the HH131 genotype, and both fragments for the HR131 genotype. FcγRIIIB-NA1/NA2 genotyping was carried out using allele-specific primers as previously described (Bux et al. 1995).
Determination of circulating IFN-γ levels
Plasma samples were obtained from venous blood and stored at −80°C until use. IFN-γ concentrations were determined using the Cytokine 25-plex Ab Bead Kit, Hu (BioSource™ International) according to the manufacturer’s protocol. Plates were read on a Luminex 100™ system (Luminex Corporation) and analyzed using the Bio-plex Manager Software (Biorad Laboratories). The detection limit for IFN-γ was 5.0 pg/mL.
Longitudinal follow-up
Children presenting for their first ‘hospital contact’ for treatment of febrile illnesses were enrolled into the SMA study at SDH (Day 0). Even though the study included children that were presenting at the Outpatient Department at SDH for routine vaccinations, these were not included in the current analyses. Parents/guardians were requested to return with their child every 3 months throughout the 3-year follow-up period. Since we determined the location of each child’s residence with our GIS surveillance system, if the parent/guardian had not returned to hospital by 1:00 p.m. for the scheduled quarterly follow-up visit, our study staff visited the child’s residence to check on their health status. In addition, since children experience multiple episodes of malaria, and other pediatric infectious diseases in this region, parents/guardians were requested to return to hospital during their child’s febrile episode(s). At each acute and quarterly visit, all laboratory tests required for proper clinical management of the patients were performed, including complete hematological indices, malaria parasitemia determination, and evaluation of bacteremia (if clinically indicated). In addition, mortality data were collected throughout the 3-year follow-up. Mortality data, along with clinical and laboratory measures for multiple episodes of malaria, were used to evaluate the association between haplotypes and longitudinal outcomes of malaria and mortality.
Statistical analyses
Statistical analyses were performed using SPSS (Version 15.0). Chi-square analyses were used to examine differences between the proportions. Across-group comparisons of non-parametric data were determined by Kruskal–Wallis tests, while Mann–Whitney U tests were used for pairwise comparisons. Deviations from Hardy–Weinberg Equilibrium (HWE) were tested using the web-based site www.tufts.edu/~mcourt01/Documents/Court%20lab%20-%20HW%20calculator.xls. Haplotypes were constructed using the HPlus software program (Fred Hutchinson Cancer Research Center) and their frequencies estimated based on a Bayesian algorithm. For the cross-sectional analyses in children with acute malaria, the associations between the FcγR (FcγRIIA-131H/R and FcγRIIIB-NA1/NA2) haplotypes and SMA were determined by multivariate logistic regression, controlling for the potential confounding effects of age, gender, HIV-1 status [including both HIV-1 exposed and definitively HIV-1(+) results], bacteremia, sickle cell trait (HbAS), and G6PD deficiency. Hierarchical logistic regression analyses were used for determining the longitudinal relationship between FcγR (FcγRIIA-131H/R and FcγRIIIB-NA1/NA2) haplotypes, SMA, and mortality. Covariates (i.e., age, gender, HIV-1 status, bacteremia, sickle cell trait, and G6PD deficiency) were entered into block 1 and the haplotype contrast (carrier vs. non-carrier) was entered into block 2. All values of P < 0.100 were further analyzed using Cox regression/survival analysis, and differences in the distributions of hazard rate functions (i.e., the probabilities of experiencing the event) between the carriers and non-carriers were examined using Mann–Whitney U tests. Significance was set at P ≤ 0.05.
Results
Demographic, clinical, and laboratory characteristics of the study participants upon enrollment
Since we hypothesized that genetic variation in the FcγR gene (i.e., FcγRIIA-131H/R and FcγRIIIB-NA1/NA2) would condition disease severity, we first performed cross-sectional analyses in children (aged <3–36 months, n = 528) presenting at hospital with acute P. falciparum malaria. Table 1 presents the demographic, clinical, and laboratory characteristics of the study participants stratified into non-severe malarial anemia (non-SMA; Hb ≥ 6.0 g/dL; n = 298) and severe malarial anemia (SMA, Hb < 6.0 g/dL; n = 230). The distribution of gender was comparable between the two groups (P = 0.367). Upon presentation at hospital, children with SMA were younger than those with non-SMA (P < 0.001). Axillary temperature (°C) was also comparable between the groups (P = 0.144). As expected, based on the a priori grouping, Hb concentrations (g/dL) and red blood cell (RBC) counts (×1012/L) were lower in the SMA group (P < 0.001 for both comparisons). White blood cell (WBC) counts (×103/μL) were elevated in the SMA group (P < 0.001). Parasite density (μL) and the prevalence of high-density parasitemia (HDP, ≥10,000 parasites/μL) were comparable between the groups (P = 0.794 and P = 0.229, respectively).
Relationship between circulating IFN-γ and disease severity
Prior to investigating the influence of polymorphic variability in FcγR on SMA, the relationship between circulating IFN-γ and disease severity was determined in children with acute malaria. As shown in Fig. 1, children with SMA had significantly higher IFN-γ plasma concentrations [median (IQR); 14.72 (12.66–48.39)] compared to the non-SMA group [7.98 (5.00-29.93), P=0.006; Fig. 1], suggesting that high levels of circulating IFN-γ are associated with enhanced disease severity in this population of children.
Association between circulating IFN-γ and SMA (Hb > 6.0 g/dL). Association between IFN-γ levels and disease severity in parasitemic children with SMA (n = 136) and non-SMA (n = 126). Boxes represent the interquartile range, the line through the box is the median, and whiskers show 10th and 90th percentiles. Statistical significance was determined by the Mann–Whitney U test. Presence of SMA was significantly associated with lower IFN-γ levels relative to non-SMA (P = 0.006)
Distribution of FcγR genotypes
After establishing that elevated circulating IFN-γ levels were significantly higher in children with SMA, the distribution of FcγRIIA-131H/R and FcγRIIIB-NA1/NA2 genotypes was determined in the population. Genotypic distributions of FcγRIIA-131H/R and FcγRIIIB-NA1/NA2 variants in the non-SMA (n=298) and SMA (n=230) groups are presented in Table 2. The prevalence of FcγRIIA-131H/R genotypes in the population was 28.2% H/H, 46.4% H/R, and 25.4% R/R, with overall allele frequencies of 0.51 for 131H and 0.49 for 131R. There was no significant departure from Hardy–Weinberg Equilibrium (HWE) in the overall cohort for FcγRIIA-131H/R (χ2 = 2.67, P = 0.101). The genotypic distribution of FcγRIIA-131H/R in the non-SMA group was 29.9% H/H, 46.3% H/R, and 23.8% R/R, yielding allele frequencies of 0.53 for 131H and 0.47 for 131R. The non-SMA group did not have significant departure from HWE (χ2 = 1.47, P = 0.224) in the FcγRIIA-131H/R gene. Distributions of genotypes in the SMA group were 26.1% H/H, 46.5% H/R, and 27.4% R/R, with allele frequencies of 0.54 for 131H and 0.46 for 131R. The SMA also failed to display significant departure from HWE (χ2 = 1.10, P = 0.292).
The prevalence of FcγRIIIB-NA1/NA2 genotypes in the population was 4.3% NA1/NA1, 65.3% NA1/NA2, and 30.4% NA2/NA2, with overall allele frequencies of 0.37 for NA1 and 0.63 for NA2. There was significant departure from HWE in the overall cohort for FcγRIIIB-NA1/NA2 (χ2 = 84.96, P < 0.001). The genotypic distribution of FcγRIIIB-NA1/NA2 in the non-SMA group was 4.6% NA1/NA1, 62.8% NA1/NA2, and 32.6% NA2/NA2, yielding allele frequencies of 0.37 for NA1 and 0.63 for NA2. The non-SMA group had significant departure from HWE (χ2 = 38.74, P < 0.001). Distributions of genotypes in the SMA group were 3.9% NA1/NA1, 68.7% NA1/NA2, and 27.4% NA2/NA2, with allele frequencies of 0.37 for NA1 and 0.63 for NA2. The SMA group also displayed significant departure from HWE (χ2 = 47.42, P < 0.001).
Further analysis revealed that the cross-sectional distribution of the FcγRIIA-131H/R and FcγRIIIB-NA1/NA2 genotypes was comparable between the non-SMA and SMA groups (P = 0.520 and P = 0.363, respectively) (Table 2).
Cross-sectional distribution of FcγR haplotypes
Construction of FcγRIIA-131H/R and FcγRIIIB-NA1/NA2 haplotypes revealed the following prevalence: FcγRIIA-131H/FcγRIIIB-NA1, 52.4% (277/528); FcγRIIA-131H/FcγRIIIB-NA2, 41.0% (217/528); FcγRIIA-131R/FcγRIIIB-NA1, 19.1% (101/528), and FcγRIIA-131R/FcγRIIIB-NA2, 69.1% (365/528). The distributions of haplotypes in the non-SMA and SMA groups are shown in Table 3. Although none of the haplotypic distributions were significantly different between the two clinical groups, there was a trend toward greater carriage of the FcγRIIA-131R/FcγRIIIB-NA2 haplotype in children with SMA (P = 0.077).
Association between FcγR haplotypes and SMA in children with acute malaria
To determine the role of FcγRIIA-131H/R and FcγRIIIB-NA1/NA2 haplotypes in conditioning susceptibility to SMA, multivariate logistic regression analyses were conducted while controlling for the potential confounding effects of age, gender, HIV-1 status, bacteremia, sickle-cell trait and G6PD deficiency (Aidoo et al. 2002; Berkley et al. 1999; Otieno et al. 2006; Wambua et al. 2006a, b). As shown in Table 4, although none of the haplotypes were significantly associated with susceptibility to SMA, the FcγRIIA-131H/FcγRIIIB-NA1 haplotype showed a trend toward a protective effect (OR 0.83, 95% CI 0.72–1.06; P = 0.088), while carriage of the FcγRIIA-131R/FcγRIIIB-NA2 haplotype was associated with enhanced risk of SMA (OR 1.44, 95% CI 0.97–2.13; P = 0.071).
Longitudinal distribution of FcγR haplotypes
Construction of FcγRIIA-131H/R and FcγRIIIB-NA1/NA2 haplotypes in those with SMA in the longitudinal follow-up revealed the following prevalence: FcγRIIA-131H/FcγRIIIB-NA1, 38.5%; FcγRIIA-131H/FcγRIIIB-NA2, 69.7%; FcγRIIA-131R/FcγRIIIB-NA1, 60.3%, and FcγRIIA-131R/FcγRIIIB-NA2, 45.4%.
Relationship between FcγR haplotypes and longitudinal outcomes
After determining the association between the haplotypes and susceptibility to SMA in children with acute malaria, we examined the relationship between carriage of the different haplotypes and longitudinal outcomes (i.e., repeated episodes of SMA and mortality) over a 3-year follow-up period. Hierarchical logistic regression analyses, controlling for age, gender, HIV-1 status, bacteremia, sickle cell trait and G6PD deficiency demonstrated that carriage of the FcγRIIA-131H/FcγRIIIB-NA1 haplotype was associated with a 35% reduced risk of developing SMA (RR 0.65, 95% CI 0.46–0.90; P = 0.012). In contrast, carriage of the FcγRIIA-131H/FcγRIIIB-NA2 haplotype was associated with an increased risk of SMA (RR 1.47, 95% CI 1.06–2.04; P = 0.020; Table 5). Given that heterozygous individuals can have a diluting effect on haplotypes, we constructed additional haplotypes based on homozygosity on the two loci (i.e., FcγRIIA-131H/H/FcγRIIIB-NA1/NA1 and FcγRIIA-131H/H/FcγRIIIB-NA2/NA2) for the haplotypes that demonstrated significant associations with SMA. Consistent with our earlier observation, this model demonstrated that the FcγRIIA-131H/H/FcγRIIIB-NA1/NA1 was associated with reduced risk to SMA (RR 0.45, 95% CI 0.37–0.74; P = 0.007) while carriage of FcγRIIA-131H/H/FcγRIIIB-NA2/NA2 was associated with an increased risk of SMA (RR 1.64, 95% CI 1.10–2.17; P = 0.016). Furthermore, haplotypes were constructed for individuals both heterozygous at FcγRIIA-131H/R and FcγRIIIB-NA1/NA2 loci. Results revealed that the presence of heterozygosity at these two loci was associated with protection against SMA (RR 0.72, 95% CI 0.48–0.93; P = 0.037) relative to those non-heterozygous, thus masking the increased susceptibility associated with FcγRIIA-131H/FcγRIIIB-NA2 haplotype.
Longitudinal analyses revealed that non-carriers of the 131H/NA1 haplotype had an increased risk to mortality relative to carriers. Consistent with these results, Cox regression modeling (controlling for covariates) revealed a higher risk of all-cause mortality in non-carriers of the 131H/NA1 haplotypes relative to carriers (P = 0.002). The mortality rate did not differ between the carriers and non-carriers of the 131R/NA1 (β = 1.36, P = 0.484), 131R/NA2 (β = 1.38, P = 0.336), and 131H/NA2 (β = 0.55, P = 0.092) haplotypes.
Influence of FcγR haplotypes on in vivo IFN-γ concentrations
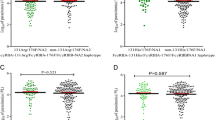
Additional analyses examined the association between FcγRIIA-131H/R and FcγRIIIB-NA1/NA2 haplotypes and circulating IFN-γ levels. Individuals with the FcγRIIA-131R/FcγRIIIB-NA1 (131R/NA1) haplotype had higher IFN-γ levels, [median (IQR) [15.45 (8.00–33.10)] relative to non-carriers [10.43 (5.00–25.42), P = 0.067], while those with the FcγRIIA-131H/FcγRIIIB-NA1 (131H/NA1) haplotype had significantly lower IFN-γ levels [8.00 (6.00–21.50)] relative to non-carriers [13.40 (9.00–35.10), P = 0.046; Fig. 2]. Circulating IFN-γ levels were comparable between the carriers of the FcγRIIA-131H/FcγRIIIB-NA2 (131H/NA2) (P = 0.551), FcγRIIA-131R/FcγRIIIB-NA2 (131R/NA2) (P = 0.708), and their respective non-carriers (Fig. 2). Further analyses tested the functional effects of co-carriage of FcγRIIA-131H/R and FcγRIIIB-NA1/NA2 alleles and circulating IFN-γ levels. Results revealed that individuals heterozygous at both loci (FcγRIIA-131H/R/FcγRIIIB-NA1/NA2) had lower IFN-γ levels, [median (IQR) [6.20 (5.00–18.40)] relative to non-carriers [12.50 (8.00–24.50), P = 0.026].
Functional association between FcγRIIA-131H/R and FcγRIIIB-NA1/NA2 haplotypes and IFN-γ. Circulating IFN-γ levels were measured using ELISA. Data are shown for the FcγRIIA-131H/FcγRIIIB-NA1 (n = 130), FcγRIIA-131H/FcγRIIIB-NA2 (n = 108), FcγRIIA-131R/FcγRIIIB-NA1 (n = 50) and FcγRIIA-131R/FcγRIIIB-NA2 (n = 185) haplotypes. FcγRIIA-131H/FcγRIIIB-NA1 = 131H/NA1 on the figure. Data are presented as box-plots, where the box represents the interquartile range, the line through the box is the median, and whiskers show the 10th and 90th percentiles. Pairwise comparisons in those with haplotype versus those without haplotypes were determined by Mann–Whitney U tests. Presence of the 131H/NA1 was associated with significantly lower circulating IFN-γ levels relative to individuals without the haplotype. In addition, even though the presence of 131R/NA1 was associated with higher production of IFN-γ levels relative to individuals without the haplotype, it was at borderline significance (P = 0.067). Shaded boxes represent presence of haplotype while open boxes represents non-haplotypes
Discussion
In the current study, we tested the hypothesis that haplotypes in FcγR genes (i.e., FcγRIIA-131H/R and FcγRIIIB-NA1/NA2) could alter susceptibility to SMA in children resident in a P. falciparum holoendemic transmission region of western Kenya. In addition, we explored whether or not these haplotypes were associated with functionality in production of IFN-γ. Cross-sectional analyses in children with acute malaria showed that the FcγRIIA-131H/R and FcγRIIIB-NA1/NA2 haplotypes did not significantly influence susceptibility to SMA. However, longitudinal analyses over a 3-year follow-up period demonstrated that carriage of the FcγRIIA-131H/FcγRIIIB-NA1 haplotype was associated with 35% reduction in the development of SMA, while the presence of FcγRIIA-131H/FcγRIIIB-NA2 haplotype increased the risk of SMA. In addition, Cox regression modeling of the follow-up data revealed a higher risk of all-cause mortality in non-carriers of the FcγRIIA-131H/FcγRIIIB-NA1 haplotypes relative to carriers. Measurement of circulating IFN-γ showed that the ‘protective’ FcγRIIA-131H/FcγRIIIB-NA1 haplotype was also associated with decreased IFN-γ production. To our knowledge, this is the first report delineating the associations between FcγRIIA-131H/R and FcγRIIIB-NA1/NA2 haplotypes, SMA (Hb < 6.0 g/dL), and mortality in holoendemic P. falciparum transmission areas, such as western Kenya. Pertinent to our observations was the fact that there were no associations between the haplotypes and SMA in the cross-sectional analyses performed in children with acute malaria, while the same haplotypes predicted susceptibility to SMA (and mortality) over a 3-year longitudinal follow-up, underscoring the importance of using longitudinal data in disease association studies.
Previous cross-sectional analyses in a large cohort in The Gambia revealed that the FcγRIIA-H/H131 genotype was associated with increased malaria disease severity, particularly in children less than 5 years of age (Cooke et al. 2003). Although severe malaria in this population was defined by a mixed phenotype of disease (CM, SMA, and/or hypoglycemia), the FcγRIIA-H/H131 genotype was more prevalent in those with SMA compared with aparasitemic children (Cooke et al. 2003). Other studies, carried out in Gedaref State in the Daraweesh population (aged 2–55 years) of eastern Sudan, demonstrated that the FcγRIIA-H/H131 genotype was more prevalent in the Fulani (who are less affected by clinical malaria) than in sympatric non-Fulani ethnic groups (Nasr et al. 2007). Additional studies demonstrated an association between the R/R131 genotype and susceptibility to severe malaria in Sudanese patients presenting with a mixed clinical phenotype (Nasr et al. 2007), while in an Indian population, the FcγRIIA-H/H131 genotype was significantly associated with protection from malaria in individuals over 5 years of age with a diverse phenotypic definition of disease (CM and/or severe anemia, acidotic breathing, pulmonary edema, hypoglycemia or increased serum creatinine levels) (Sinha et al. 2008). Our previous studies (Ouma et al. 2006) and those of others in adjacent areas of western Kenya (Shi et al. 2001) in children with malaria demonstrated that the FcγRIIA-R/R131 genotype was associated with protection against HDP and lower thresholds of parasitemia. A previous cross-sectional study in Thai adults showed that the FcγRIIA-H/H131 genotype, together with the FcγRIIIB-NA2 allele, was associated with increased susceptibility to CM (Omi et al. 2002). Our haplotypic findings demonstrated that carriage of the FcγRIIA-131H/FcγRIIIB-NA1 haplotype was associated with a reduced risk of SMA, while carriers of the FcγRIIA-131H/FcγRIIIB-NA2 haplotype had an increased risk of developing SMA. Reasons for differences in results presented here versus previous findings may be related to the following: (a) there are likely diverse immunoprotective responses to malaria conditioned by different exposure rates (i.e., hyper- vs. holoendemic); (b) since CM is a rare manifestation of severe malaria in our cohort and most of the previous studies included this disease phenotype, the potentially powerful effects of the FcγR genotypes on CM cannot be observed in a population in which the primary clinical outcome of severe malaria is SMA; and c) previous studies focused on single polymorphisms, while in the current study, we investigated the associations between haplotypes and susceptibility to SMA. Results presented here underscore the fact that multi-site haplotypes are highly informative allelic markers that can reveal associations with disease outcomes not identifiable with single polymorphisms (Ouma et al. 2008a, b).
FcγRs on macrophages/monocytes play an important role in the pathogenesis of severe malaria by mediating phagocytosis of both infected and uninfected RBCs and by triggering the production of pro-inflammatory cytokines. For example, previous studies demonstrated that TNF-α secretion by these cell populations varied considerably among individuals after FcγR cross-linking (Debets et al. 1988). Additional analyses demonstrated that clearance half-lives of IgG-sensitized RBCs varied in patients presenting with acute P. falciparum malaria and was directly correlated with hematocrit values (Ho et al. 1990; Lee et al. 1989), implying that FcγR-mediated clearance of uninfected erythrocytes may be important in the development of SMA. These observations, coupled with findings showing heterogeneity of responses to stimulatory signals among monocytes from different individuals, suggest that individuals with responses that lead to enhanced erythrophagocytic activity and/or production of high levels of TNF-α may be predisposed to SMA (Ogonda et al. 2010). We present findings in the current study showing that FcγRIIA-131H/FcγRIIIB-NA1 haplotype was associated with a decreased risk of SMA, while carriage of FcγRIIA-131H/FcγRIIIB-NA2 haplotype increased the risk of SMA during a 3-year follow-up period. Additional investigation of the relationship between FcγR haplotypes and susceptibility to mortality over the 3-year follow-up demonstrated that non-carriers of FcγRIIA-131H/FcγRIIIB-NA1 haplotype had a higher risk of all-cause mortality relative to carriers. This observation is consistent with the fact that carriers of the FcγRIIA-131H/FcγRIIIB-NA1 haplotype had a significantly reduced risk of SMA over the same period. These results further illustrate that the same FcγR haplotypes that are important for conditioning susceptibility to repeated episodes of SMA also influence the risk of mortality during the critical phases in which naturally acquired malarial immunity is developing.
There is accumulating evidence about the role of IFN-γ in the control of infectious diseases. Earlier studies carried out in a malaria endemic region of Ghana demonstrated that production of malaria-specific IFN-γ was associated with the reduced risk of fever and clinical malaria (Dodoo et al. 2002). Additional studies revealed that in cases of uncomplicated malaria, activated CD8 T cells produce IFN-γ through macrophage activation (Torre et al. 2002b), a process that may be important in limiting parasite expansion and, thereby preventing disease progression toward severe, life-threatening malaria (Torre et al. 2002a). Recent studies in Nigerian children less than 6 months of age showed higher levels of IFN-γ in symptomatic children relative to non-symptomatic controls, with the interpretation that elevated IFN-γ levels were instrumental in immune-mediated protection against malaria by limiting parasite replication (Iriemenam et al. 2009). Exposure to IFN-γ in a variety of tissues and cells significantly increases the transcriptional (or post-transcriptional) expression of several major histocompatibility complex (MHC) class I genes, including FcγR (Colgan et al. 1996; Gobin et al. 1999; Lefebvre et al. 1999; Yang et al. 1996). Elevated expression of FcγR can further augment binding of cytophilic immunoglobulins leading to enhanced phagocytosis by monocytes/macrophages. Although collectively these studies illustrate the importance IFN-γ in controlling parasitemia, the precise role of IFN-γ in the pathogenesis of SMA remains elusive. For example, in the current study, we demonstrate that children with SMA had significantly higher circulating IFN-γ levels, and that the FcγRIIA-131H/FcγRIIIB-NA1 haplotype, which protected against SMA, was characterized by significantly lower IFN-γ production. Increased IFN-γ production can promote elevated TNF-α levels (Urban et al. 2005) which have been shown to enhance the pathogenesis of SMA (Kurtzhals et al. 1999; Luty et al. 2000; McDevitt et al. 2004; Othoro et al. 1999; Prakash et al. 2006). As such, although not explored in the current study, it is hypothesized that lower IFN-γ levels in children with FcγRIIA-131H/FcγRIIIB-NA1 haplotype may prevent overproduction of TNF-α, thereby leading to a decreased risk for development of SMA.
Studies show an inverse correlation between IFN-γ and parasite density supporting the notion that IFN-γ plays a protective role against parasitemia (D’Ombrain et al. 2008; McCall et al. 2010; Pombo et al. 2002; Walther et al. 2006). However, it is important to note that childhood SMA in holoendemic transmission regions is typically not defined by higher levels of peripheral parasitemia (Ong’echa et al. 2006). We hypothesize that elevated levels of IFN-γ are important for controlling parasitemia during the early phases of infection, but that persistently high levels, and/or the inappropriate timing of IFN-γ release (conditioned by genetic variation) may promote pathological consequences, such as SMA. Although confirmation of this hypothesis will require a time-series analysis of a panel of immune mediators (IFN-γ included) during different phases of the immune response to malaria in individuals stratified according to haplotypic groups, previous studies demonstrated that generation of pro-inflammatory cytokines that control malaria parasitemia may also contribute to the immunopathology of disease (Artavanis-Tsakonas et al. 2003).
In conclusion, we show that the reduced susceptibility to repeated episodes of SMA and mortality over the critical time periods in which naturally acquired immunity to malaria is developing is associated with haplotypes in the FcγR gene which produce lower levels of IFN-γ responses. Future studies aimed at further defining how genetic variation conditions IFN-γ responses may aid in defining important correlates of protection against SMA that can be used in vaccine development and testing.
References
Aidoo M, Terlouw DJ, Kolczak MS, McElroy PD, ter Kuile FO, Kariuki S, Nahlen BL, Lal AA, Udhayakumar V (2002) Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet 359:1311–1312
Artavanis-Tsakonas K, Tongren JE, Riley EM (2003) The war between the malaria parasite and the immune system: immunity, immunoregulation and immunopathology. Clin Exp Immunol 133:145–152
Berkley JA, Mwangi I, Mellington F, Mwarumba S, Marsh K (1999) Cerebral malaria versus bacterial meningitis in children with impaired consciousness. QJM 92:151–157
Bloland PB, Boriga DA, Ruebush TK, McCormick JB, Roberts JM, Oloo AJ, Hawley W, Lal A, Nahlen B, Campbell CC (1999a) Longitudinal cohort study of the epidemiology of malaria infections in an area of intense malaria transmission II. Descriptive epidemiology of malaria infection and disease among children. Am J Trop Med Hyg 60:641–648
Bloland PB, Ruebush TK, McCormick JB, Ayisi J, Boriga DA, Oloo AJ, Beach R, Hawley W, Lal A, Nahlen B, Udhayakumar V, Campbell CC (1999b) Longitudinal cohort study of the epidemiology of malaria infections in an area of intense malaria transmission I. Description of study site, general methodology, and study population. Am J Trop Med Hyg 60:635–640
Bouharoun-Tayoun H, Oeuvray C, Lunel F, Druilhe P (1995) Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J Exp Med 182:409–418
Bux J, Stein EL, Santoso S, Mueller-Eckhardt C (1995) NA gene frequencies in the German population, determined by polymerase chain reaction with sequence-specific primers. Transfusion 35:54–57
Chehimi J, Trinchieri G (1994) Interleukin-12: a bridge between innate resistance and adaptive immunity with a role in infection and acquired immunodeficiency. J Clin Immunol 14:149–161
Colgan SP, Morales VM, Madara JL, Polischuk JE, Balk SP, Blumberg RS (1996) IFN-gamma modulates CD1d surface expression on intestinal epithelia. Am J Physiol 271:C276–C283
Cooke GS, Aucan C, Walley AJ, Segal S, Greenwood BM, Kwiatkowski DP, Hill AV (2003) Association of Fcgamma receptor IIa (CD32) polymorphism with severe malaria in West Africa. Am J Trop Med Hyg 69:565–568
Debets JM, Van der Linden CJ, Dieteren IE, Leeuwenberg JF, Buurman WA (1988) Fc-receptor cross-linking induces rapid secretion of tumor necrosis factor (cachectin) by human peripheral blood monocytes. J Immunol 141:1197–1201
Dodoo D, Omer FM, Todd J, Akanmori BD, Koram KA, Riley EM (2002) Absolute levels and ratios of proinflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to Plasmodium falciparum malaria. J Infect Dis 185:971–979
D’Ombrain MC, Robinson LJ, Stanisic DI, Taraika J, Bernard N, Michon P, Mueller I, Schofield L (2008) Association of early interferon-gamma production with immunity to clinical malaria: a longitudinal study among Papua New Guinean children. Clin Infect Dis 47:1380–1387
English M, Wale S, Binns G, Mwangi I, Sauerwein H, Marsh K (1998) Hypoglycaemia on and after admission in Kenyan children with severe malaria. QJM 91:191–197
Farouk SE, Dolo A, Bereczky S, Kouriba B, Maiga B, Farnert A, Perlmann H, Hayano M, Montgomery SM, Doumbo OK, Troye-Blomberg M (2005) Different antibody- and cytokine-mediated responses to Plasmodium falciparum parasite in two sympatric ethnic tribes living in Mali. Microbes Infect 7:110–117
Gajewski TF, Joyce J, Fitch FW (1989) Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. J Immunol 143:15–22
Gobin SJ, van Zutphen M, Woltman AM, van den Elsen PJ (1999) Transactivation of classical and nonclassical HLA class I genes through the IFN-stimulated response element. J Immunol 163:1428–1434
Ho M, White NJ, Looareesuwan S, Wattanagoon Y, Lee SH, Walport MJ, Bunnag D, Harinasuta T (1990) Splenic Fc receptor function in host defense and anemia in acute Plasmodium falciparum malaria. J Infect Dis 161:555–561
Iriemenam NC, Okafor CM, Balogun HA, Ayede I, Omosun Y, Persson JO, Hagstedt M, Anumudu CI, Nwuba RI, Troye-Blomberg M, Berzins K (2009) Cytokine profiles and antibody responses to Plasmodium falciparum malaria infection in individuals living in Ibadan, southwest Nigeria. Afr Health Sci 9:66–74
Janeway CA Jr, Medzhitov R (2002) Innate immune recognition. Annu Rev Immunol 20:197–216
Jiang XM, Arepally G, Poncz M, McKenzie SE (1996) Rapid detection of the Fc gamma RIIA-H/R 131 ligand-binding polymorphism using an allele-specific restriction enzyme digestion (ASRED). J Immunol Methods 199:55–59
Kobayashi F, Ishida H, Matsui T, Tsuji M (2000) Effects of in vivo administration of anti-IL-10 or anti-IFN-gamma monoclonal antibody on the host defense mechanism against Plasmodium yoelii yoelii infection. J Vet Med Sci 62:583–587
Kurtzhals JA, Akanmori BD, Goka BQ, Adabayeri V, Nkrumah FK, Behr C, Hviid L (1999) The cytokine balance in severe malarial anemia. J Infect Dis 180:1753–1755
Lee SH, Looareesuwan S, Wattanagoon Y, Ho M, Wuthiekanun V, Vilaiwanna N, Weatherall DJ, White NJ (1989) Antibody-dependent red cell removal during P. falciparum malaria: the clearance of red cells sensitized with an IgG anti-D. Br J Haematol 73:396–402
Lefebvre S, Moreau P, Guiard V, Ibrahim EC, Adrian-Cabestre F, Menier C, Dausset J, Carosella ED, Paul P (1999) Molecular mechanisms controlling constitutive and IFN-gamma-inducible HLA-G expression in various cell types. J Reprod Immunol 43:213–224
Luty AJ, Perkins DJ, Lell B, Schmidt-Ott R, Lehman LG, Luckner D, Greve B, Matousek P, Herbich K, Schmid D, Weinberg JB, Kremsner PG (2000) Low interleukin-12 activity in severe Plasmodium falciparum malaria. Infect Immun 68:3909–3915
McCall MB, Hopman J, Daou M, Maiga B, Dara V, Ploemen I, Nganou-Makamdop K, Niangaly A, Tolo Y, Arama C, Bousema JT, van der Meer JW, van der Ven AJ, Troye-Blomberg M, Dolo A, Doumbo OK, Sauerwein RW (2010) Early interferon-gamma response against Plasmodium falciparum correlates with interethnic differences in susceptibility to parasitemia between sympatric Fulani and Dogon in Mali. J Infect Dis 201:142–152
McDevitt MA, Xie J, Gordeuk V, Bucala R (2004) The anemia of malaria infection: role of inflammatory cytokines. Curr Hematol Rep 3:97–106
McElroy PD, Lal AA, Hawley WA, Bloland PB, Kuile FO, Oloo AJ, Harlow SD, Lin X, Nahlen BL (1999) Analysis of repeated hemoglobin measures in full-term, normal birth weight Kenyan children between birth and four years of age. III. The Asemobo Bay Cohort Project. Am J Trop Med Hyg 61:932–940
Miller CH, Maher SG, Young HA (2009) Clinical use of interferon-gamma. Ann N Y Acad Sci 1182:69–79
Mockenhaupt FP, Ehrhardt S, Burkhardt J, Bosomtwe SY, Laryea S, Anemana SD, Otchwemah RN, Cramer JP, Dietz E, Gellert S, Bienzle U (2004) Manifestation and outcome of severe malaria in children in northern Ghana. Am J Trop Med Hyg 71:167–172
Nasr A, Iriemenam NC, Troye-Blomberg M, Giha HA, Balogun HA, Osman OF, Montgomery SM, ElGhazali G, Berzins K (2007) Fc gamma receptor IIa (CD32) polymorphism and antibody responses to asexual blood-stage antigens of Plasmodium falciparum malaria in Sudanese patients. Scand J Immunol 66:87–96
Ogonda LA, Orago AS, Otieno MF, Adhiambo C, Otieno W, Stoute JA (2010) The levels of CD16/Fc gamma receptor IIIA on CD14+ CD16+ monocytes are higher in children with severe Plasmodium falciparum anemia than in children with cerebral or uncomplicated malaria. Infect Immun 78:2173–2181
Omi K, Ohashi J, Patarapotikul J, Hananantachai H, Naka I, Looareesuwan S, Tokunaga K (2002) Fcgamma receptor IIA and IIIB polymorphisms are associated with susceptibility to cerebral malaria. Parasitol Int 51:361–366
Ong’echa JM, Keller CC, Were T, Ouma C, Otieno RO, Landis-Lewis Z, Ochiel D, Slingluff JL, Mogere S, Ogonji GA, Orago AS, Vulule JM, Kaplan SS, Day RD, Perkins DJ (2006) Parasitemia, anemia, and malarial anemia in infants and young children in a rural holoendemic Plasmodium falciparum transmission area. Am J Trop Med Hyg 74:376–385
Othoro C, Lal AA, Nahlen B, Koech D, Orago AS, Udhayakumar V (1999) A low interleukin-10 tumor necrosis factor-alpha ratio is associated with malaria anemia in children residing in a holoendemic malaria region in western Kenya. J Infect Dis 179:279–282
Otieno RO, Ouma C, Ong’echa JM, Keller CC, Were T, Waindi EN, Michaels MG, Day RD, Vulule JM, Perkins DJ (2006) Increased severe anemia in HIV-1-exposed and HIV-1-positive infants and children during acute malaria. AIDS 20:275–280
Ouma C, Keller CC, Opondo DA, Were T, Otieno RO, Otieno MF, Orago AS, Ong’Echa JM, Vulule JM, Ferrell RE, Perkins DJ (2006) Association of FCgamma receptor IIA (CD32) polymorphism with malarial anemia and high-density parasitemia in infants and young children. Am J Trop Med Hyg 74:573–577
Ouma C, Davenport GC, Awandare GA, Keller CC, Were T, Otieno MF, Vulule JM, Martinson J, Ong’echa JM, Ferrell RE, Perkins DJ (2008a) Polymorphic variability in the interleukin (IL)-1beta promoter conditions susceptibility to severe malarial anemia and functional changes in IL-1beta production. J Infect Dis 198:1219–1226
Ouma C, Davenport GC, Were T, Otieno MF, Hittner JB, Vulule JM, Martinson J, Ong’echa JM, Ferrell RE, Perkins DJ (2008b) Haplotypes of IL-10 promoter variants are associated with susceptibility to severe malarial anemia and functional changes in IL-10 production. Hum Genet 124:515–524
Ouma C, Keller CC, Davenport GC, Were T, Konah S, Otieno MF, Hittner JB, Vulule JM, Martinson J, Ong’echa JM, Ferrell RE, Perkins DJ (2010) A novel functional variant in the stem cell growth factor promoter protects against severe malarial anemia. Infect Immun 78:453–460
Pombo DJ, Lawrence G, Hirunpetcharat C, Rzepczyk C, Bryden M, Cloonan N, Anderson K, Mahakunkijcharoen Y, Martin LB, Wilson D, Elliott S, Eisen DP, Weinberg JB, Saul A, Good MF (2002) Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet 360:610–617
Prakash D, Fesel C, Jain R, Cazenave PA, Mishra GC, Pied S (2006) Clusters of cytokines determine malaria severity in Plasmodium falciparum-infected patients from endemic areas of Central India. J Infect Dis 194:198–207
Sabchareon A, Burnouf T, Ouattara D, Attanath P, Bouharoun-Tayoun H, Chantavanich P, Foucault C, Chongsuphajaisiddhi T, Druilhe P (1991) Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg 45:297–308
Salmon JE, Edberg JC, Kimberly RP (1990) Fc gamma receptor III on human neutrophils. Allelic variants have functionally distinct capacities. J Clin Invest 85:1287–1295
Shi YP, Nahlen BL, Kariuki S, Urdahl KB, McElroy PD, Roberts JM, Lal AA (2001) Fcgamma receptor IIa (CD32) polymorphism is associated with protection of infants against high-density Plasmodium falciparum infection. VII. Asembo Bay Cohort Project. J Infect Dis 184:107–111
Sinha S, Mishra SK, Sharma S, Patibandla PK, Mallick PK, Sharma SK, Mohanty S, Pati SS, Ramteke BK, Bhatt R, Joshi H, Dash AP, Ahuja RC, Awasthi S, Venkatesh V, Habib S (2008) Polymorphisms of TNF-enhancer and gene for FcgammaRIIa correlate with the severity of falciparum malaria in the ethnically diverse Indian population. Malar J 7:13
Stein MP, Edberg JC, Kimberly RP, Mangan EK, Bharadwaj D, Mold C, Du Clos TW (2000) C-reactive protein binding to FcgammaRIIa on human monocytes and neutrophils is allele-specific. J Clin Invest 105:369–376
Tangteerawatana P, Pichyangkul S, Hayano M, Kalambaheti T, Looareesuwan S, Troye-Blomberg M, Khusmith S (2007) Relative levels of IL4 and IFN-gamma in complicated malaria: association with IL4 polymorphism and peripheral parasitemia. Acta Trop 101:258–265
Torre D, Speranza F, Giola M, Matteelli A, Tambini R, Biondi G (2002a) Role of Th1 and Th2 cytokines in immune response to uncomplicated Plasmodium falciparum malaria. Clin Diagn Lab Immunol 9:348–351
Torre D, Speranza F, Martegani R (2002b) Role of proinflammatory and anti-inflammatory cytokines in the immune response to Plasmodium falciparum malaria. Lancet Infect Dis 2:719–720
Urban BC, Ing R, Stevenson MM (2005) Early interactions between blood-stage plasmodium parasites and the immune system. Curr Top Microbiol Immunol 297:25–70
Vignoli C, de Lamballerie X, Zandotti C, Tamalet C, de Micco P (1995) Advantage of a rapid extraction method of HIV1 DNA suitable for polymerase chain reaction. Res Virol 146:159–162
Walther M, Woodruff J, Edele F, Jeffries D, Tongren JE, King E, Andrews L, Bejon P, Gilbert SC, De Souza JB, Sinden R, Hill AV, Riley EM (2006) Innate immune responses to human malaria: heterogeneous cytokine responses to blood-stage Plasmodium falciparum correlate with parasitological and clinical outcomes. J Immunol 177:5736–5745
Wambua S, Mwacharo J, Uyoga S, Macharia A, Williams TN (2006a) Co-inheritance of alpha+-thalassaemia and sickle trait results in specific effects on haematological parameters. Br J Haematol 133:206–209
Wambua S, Mwangi TW, Kortok M, Uyoga SM, Macharia AW, Mwacharo JK, Weatherall DJ, Snow RW, Marsh K, Williams TN (2006b) The effect of alpha+-thalassaemia on the incidence of malaria and other diseases in children living on the coast of Kenya. PLoS Med 3:e158
Warmerdam PA, van de Winkel JG, Vlug A, Westerdaal NA, Capel PJ (1991) A single amino acid in the second Ig-like domain of the human Fc gamma receptor II is critical for human IgG2 binding. J Immunol 147:1338–1343
Were T, Davenport GC, Hittner JB, Ouma C, Vulule JM, Ong’echa JM, Perkins DJ (2011) Bacteremia in Kenyan children presenting with malaria. J Clin Microbiol 49: 671–676
Yang Y, Chu W, Geraghty DE, Hunt JS (1996) Expression of HLA-G in human mononuclear phagocytes and selective induction by IFN-gamma. J Immunol 156:4224–4231
Acknowledgments
We are grateful to the Siaya District Hospital team and the University of New Mexico/KEMRI staff for support and management: Nicholas Otieno and Micheal Odhiambo for clinical support; Tabitha Otieno, Chris Wasonga, Joan Ochieng, and Godfrey Ogulla for laboratory support; Vincent Omanje, Jared Ondijo, Brenda Sudi and Caroline Odette for data management; Rodney Bosire for field support; and Anne On’gondo for administrative support. We are also grateful to the parents/guardians of the study participants and the children that participated in the study. These data are published with the approval of the Director, Kenya Medical Research Institute (KEMRI). This work was supported by grants from the National Institute of Health [TW008306-02 (CO)], [AI51305-02 (DJP) and TW05884-02 (DJP)]. The content of this publication is the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health. The study was approved by the ethical and scientific review committees at the Kenya Medical Research Institute and the institutional review board at the University of Pittsburgh and University of New Mexico.
Conflict of interest
There is no conflict of interest for any of the authors of the manuscript due to commercial or other affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ouma, C., Davenport, G.C., Garcia, S. et al. Functional haplotypes of Fc gamma (Fcγ) receptor (FcγRIIA and FcγRIIIB) predict risk to repeated episodes of severe malarial anemia and mortality in Kenyan children. Hum Genet 131, 289–299 (2012). https://doi.org/10.1007/s00439-011-1076-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-011-1076-8