Abstract
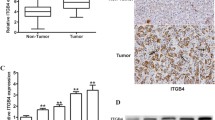
Tumor invasion and metastasis are complex processes, requiring the ability of tumor cells to interact with proteins of the extracellular matrix through cell-adhesion molecules on the cell surface. Integrins are heterodimeric membrane glycoproteins, consisting of α and β subunits, which enable cells to recognize adhesive substrates in the extracellular matrix. The roles of the integrinα 5 β 1 in tumor invasion are highlighted by finding that some tumor cells have lost or reducedα 5 β 1 expression. It therefore functions as a negative signaling regulator. Expression ofα 5 β 1 and its mediation of cell adhesion in hepatocellular carcinoma (HCC) have not been elucidated. In surgical specimens of HCC we found, by immunohistochemistry and Northern blot analysis, that theα 5-positive rates in cancerous tissues were lower than the corresponding rates in non-cancerous tissues. Reduced expression of the integrinα 5 occurred more frequently in HCC with more malignant phenotypes, such as poor differentiation, large size (more than 10-cm in diameter), absence of capsule and high invasion. Reverse transcription/polymerase chain reaction, a more sensitive assay, was used to detect theα 5 mRNA level in LCID20, a highly metastatic model of human HCC, and LCID35, a low-metastasis model. The results showed that integrinα 5 was negative in the former and positive in the latter. Cell adhesion assays showed the maximal percentage inhibition of anti-α 5 mAb on SMMC 7721 cell adhesion to fibronectin to be 68.9±4.9% at the saturation concentrations of each antibody (200 μg/ml). If anti-α 5 mAb was combined with anti-β 1 mAb, the inhibition was 74.1±11.1%. It is concluded that reduced expression of the integrinα 5 subunit is correlated with more malignant phenotypes of human HCC. Any change in the adhesion of hepatocellular carcinoma cells to fibronectin is mainly dependent upon the function of the integrinα 5 β 1.
Similar content being viewed by others
References
Albelda SM (1993) Role of integrins and other cell adhesion molecules in tumor progression and metastasis. J Lab Invest 68:4–18
Albelda SM, Mette SA, Elder DE, Stewart R, Damjanovich I, Herlyn M, Buck CA (1990) Integrin distribution in malignant melanoma: association of the β 3 subunit with tumor progression. Cancer Res 50:6757–6764
Busk M, Sheppard D (1992) Characterization of the integrin αvβ6 as a fibronectin-binding protein. J Biol Chem 267:5790–5796
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-choloroform extract. Anal Biochem 162:156–159
Feldman LE, Shin KC, Natale RB (1991)β 1 integrin expression on human small cell lung cancer cells. Cancer Res 51:1065–1070
Giancotti FG, Ruoslahti E (1990) Elevated levels of theα 5 β 1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell 60:849–859
Giancotti FG, Mainiero F (1994) Integrin-mediated adhesion and signaling in tumorigenesis. B B A 1198:47–64
Hynes RO (1992) Integrin: versatility, modulation, and signaling in cell adhesion. Cell 69:11–25
Juliano RL, Varner JA (1993) Adhesion molecules in cancer: the role of integrins. Curr Opin Cell Biol 5:812–818
Koretz K, Schlag P, Boumsell L, Moller P (1991) Expression of α2,α 5, α6 andβ 1 chains in normal mucosa and in adenomas of the colon, colon carcinomas and their liver metastases. Am J Pathol 139:741–750
Liotta LA (1986) Tumor invasion and metastasis — role of the extracellular maxtrix: Rhoads Memorial Award Lecture. Cancer Res 46:1–7
Pignetelli M, Smith MEF, Bodmer WF (1990) Low expression of collagen receptors in moderate and poorly differentiated colorectal adenocarcinoma. Br J Cancer 61:636–638
Plantefaber LC, Hynes RO (1989) Changes in integrin receptors on oncogenically transformed cells. Cell 56:281–290
Ruoslahti E (1991) Integrins. J Clin Invest 87:1–5
Schreiner C, Fisher M, Hussenin S, Juliano RI (1991) Increased tumorigenicity of fibronectin receptor deficient Chinese hamster ovary cell variants. Cancer Res 51:1738–1740
Sell S, Ruoslahti E (1982) Expression of fibronectin and laminin in the rat liver after partial hepatectomy, during carcinogenesis, and in transplantable hepatocellular carcinomas. J Natl Cancer Inst 69:1105–1114
Stallmach A, von Lampe BV, Matthes H, Bornhoft G, Riecken EO (1992) Diminished expression of integrin adhesion molecules on human colonic eptithelial cells during the benign to malignant tumor transformation. Gut 33:342–346
Stamatoglou SS, Manson MM, Green JA, Mayol X, Hughes RC (1991) Distribution of fibronectin-binding proteins, AGp110 and integrinα 5 β 1, during chemically induced hepatocarcinogenesis in adult rats. J Cell Sci 100:599–604
Sun FX, Tang ZY, Liu KD, Ye SL, Xue Q, Gao DM, Ma ZC (1996) Establishment of a metastatic model of human hepatocellular carcinoma in nude mice via orthotopic implantation of histologically intact tissues. Int J Cancer 66:239–243
Vargner JA, Fisher MH, Juliano RI (1992) Ectopic expression of integrinα 5 β 1 suppresses in vitro growth and tumorigenicity of human colon carcinoma. Mol Biol Cell 3:323a
Volpes R, Oord JJ van den, Desmet VJ (1993) Integrins as differential cell lineage markers of primary liver tumors. Am J Pathol 142:1483–1492
Weinel RJ, Rosendahi A, Neumann K, Chaloupka B, Erd D, Rothmund M, Santoso S (1992) Expression and function of VLA-α2, α3,α 5 and α6 integrin receptors in pancreatic carcinoma. Int J Cancer 52:827–833
Yamada KM, Akiyama SK, Hasegawa T (1985) Recent advances on research of fibronectin and other cell attachment proteins. J Cell Biochem 28:78–98
Zhou XD, Tang ZY, Yu YQ, Yang BH, LU JZ, Lin ZY, Ma ZC, Zhang BH (1994) Recurrence after resection of α-fetoprotein-positive hepatocellular carcinoma. J Cancer Res Clin Oncol 120:369–373
Zutter MM, Mazoujian G, Santoro SA (1990) Decreased expression of integrin adhesion protein receptors in adenocarcinoma of the breast. Am J Pathol 137:863–870
Zutter MM, Krigman HR, Santoro SA (1993) Altered integrin expression in adenocarcinoma of the breast. Am J Pathol 142:1439–1448
Author information
Authors and Affiliations
Additional information
This work is supported by a Grant of Leading Speciality by Shanghai Health Bureau, Shanghai Education Bureau and American Chinese Medical Board (no. 93583)
Rights and permissions
About this article
Cite this article
Yao, M., Zhou, XD., Zha, XL. et al. Expression of the integrinα 5 subunit and its mediated cell adhesion in hepatocellular carcinoma. J Cancer Res Clin Oncol 123, 435–440 (1997). https://doi.org/10.1007/BF01372547
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01372547