Abstract
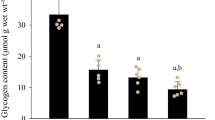
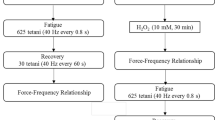
The effects of the general antioxidant N-acetylcysteine (NAC) on muscle function and metabolism were examined. Isolated paired mouse extensor digitorum longus muscles were studied in the absence or presence of 20 mM NAC. Muscles were electrically stimulated to perform 100 isometric tetanic contractions (300 ms duration) at frequencies resulting in ∼85 % of maximal force (70–150 Hz at 25–40 °C). NAC did not significantly affect peak force in the unfatigued state at any temperature but significantly slowed tetanic force development in a temperature-dependent fashion (e.g., time to 50 % of peak tension averaged 35 ± 2 ms [control] and 37 ± 1 ms [NAC] at 25 °C vs. 21 ± 1 ms [control] and 52 ± 6 ms [NAC, P < 0.01] at 40 °C). During repeated contractions, NAC maximally enhanced peak force by the fifth tetanus at all temperatures (by ∼30 %). Thereafter, the effect of NAC disappeared rapidly at high temperatures (35–40 °C) and more slowly at the lower temperatures (25–30 °C). At all temperatures, the enhancing effect of NAC on peak force was associated with a slowing of relaxation. NAC did not significantly affect myosin light chain phosphorylation at rest or after five contractions (∼50 % increase vs. rest). After five tetani, lactate and inorganic phosphate increased about 20-fold and 2-fold, respectively, both in control and NAC-treated muscles. Interestingly, after five tetani, the increase in glucose 6-P was ∼2-fold greater, whereas the increase in malate was inhibited by ∼75 % with NAC vs. control, illustrating the metabolic effects of NAC. NAC slightly decreased the maximum shortening velocity in early fatigue (five to seven repeated tetani). These data demonstrate that the antioxidant NAC transiently enhances muscle force generation by a mechanism that is independent of changes in myosin light chain phosphorylation and inorganic phosphate. The slowing of relaxation suggests that NAC enhances isometric force by facilitating fusion (i.e., delaying force decline between pulses). The initial slowing of tension development and subsequent slowing of relaxation suggest that NAC would result in impaired performance during a high-intensity dynamic exercise.





Similar content being viewed by others
References
Andersson DC, Fauconnier J, Yamada T, Lacampagne A, Zhang SJ, Katz A, Westerblad H (2011) Mitochondrial production of reactive oxygen species contributes to the beta-adrenergic stimulation of mouse cardiomyocytes. J Physiol 589:1791–1801
Andrade FH, Reid MB, Westerblad H (2001) Contractile response of skeletal muscle to low peroxide concentrations: myofibrillar calcium sensitivity as a likely target for redox-modulation. FASEB J 15:309–311
Ates B, Abraham L, Ercal N (2008) Antioxidant and free radical scavenging properties of N-acetylcysteine amide (NACA) and comparison with N-acetylcysteine (NAC). Free Radic Res 42:372–377
Barclay CJ, Lichtwark GA (2007) The mechanics of mouse skeletal muscle when shortening during relaxation. J Biomech 40:3121–3129
Bruton JD, Place N, Yamada T, Silva JP, Andrade FH, Dahlstedt AJ, Zhang SJ, Katz A, Larsson NG, Westerblad H (2008) Reactive oxygen species and fatigue-induced prolonged low-frequency force depression in skeletal muscle fibres of rats, mice and SOD2 overexpressing mice. J Physiol 586:175–184
Diaz PT, Brownstein E, Clanton TL (1994) Effects of N-acetylcysteine on in vitro diaphragm function are temperature dependent. J Appl Physiol 77:2434–2439
Edwards JN, Macdonald WA, Van der PC, Stephenson DG (2007) O2(*−) production at 37 degrees C plays a critical role in depressing tetanic force of isolated rat and mouse skeletal muscle. Am J Physiol Cell Physiol 293:C650–C660
El Awady MS, Ansari HR, Fil D, Tilley SL, Mustafa SJ (2011) NADPH oxidase pathway is involved in aortic contraction induced by A3 adenosine receptor in mice. J Pharmacol Exp Ther 338:711–717
Fedorova M, Kuleva N, Hoffmann R (2009) Reversible and irreversible modifications of skeletal muscle proteins in a rat model of acute oxidative stress. Biochim Biophys Acta 1792:1185–1193
Gordon AM, Homsher E, Regnier M (2000) Regulation of contraction in striated muscle. Physiol Rev 80:853–924
Ivy JL, Chi MM, Hintz CS, Sherman WM, Hellendall RP, Lowry OH (1987) Progressive metabolite changes in individual human muscle fibers with increasing work rates. Am J Physiol 252:C630–C639
Katz A (2007) Modulation of glucose transport in skeletal muscle by reactive oxygen species. J Appl Physiol 102:1671–1676
Khawli FA, Reid MB (1994) N-Acetylcysteine depresses contractile function and inhibits fatigue of diaphragm in vitro. J Appl Physiol 77:317–324
Koechlin C, Couillard A, Simar D, Cristol JP, Bellet H, Hayot M, Prefaut C (2004) Does oxidative stress alter quadriceps endurance in chronic obstructive pulmonary disease? Am J Respir Crit Care Med 169:1022–1027
Lamb GD, Westerblad H (2011) Acute effects of reactive oxygen and nitrogen species on the contractile function of skeletal muscle. J Physiol 589:2119–2127
Lannergren J (1978) The force-velocity relation of isolated twitch and slow muscle fibres of Xenopus laevis. J Physiol 283:501–521
Lowry OH, Passonneau JV (1972) A flexible system of enzymatic analysis. Academic, New York
Martins AS, Shkryl VM, Nowycky MC, Shirokova N (2008) Reactive oxygen species contribute to Ca2+ signals produced by osmotic stress in mouse skeletal muscle fibres. J Physiol 586:197–210
Matuszczak Y, Farid M, Jones J, Lansdowne S, Smith MA, Taylor AA, Reid MB (2005) Effects of N-acetylcysteine on glutathione oxidation and fatigue during handgrip exercise. Muscle Nerve 32:633–638
Medved I, Brown MJ, Bjorksten AR, Leppik JA, Sostaric S, McKenna MJ (2003) N-Acetylcysteine infusion alters blood redox status but not time to fatigue during intense exercise in humans. J Appl Physiol 94:1572–1582
Medved I, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X, McKenna MJ (2004) N-Acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J Appl Physiol 97:1477–1485
Mollica JP, Dutka TL, Merry TL, Lamboley CR, McConell GK, McKenna MJ, Murphy RM, Lamb GD (2012) S-Glutathionylation of troponin I (fast) increases contractile apparatus Ca2+ sensitivity in fast-twitch muscle fibres of rats and humans. J Physiol 590:1443–1463
Moopanar TR, Allen DG (2005) Reactive oxygen species reduce myofibrillar Ca2+ sensitivity in fatiguing mouse skeletal muscle at 37 degrees C. J Physiol 564:189–199
Powers SK, Jackson MJ (2008) Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88:1243–1276
Reid MB (2008) Free radicals and muscle fatigue: of ROS, canaries, and the IOC. Free Radic Biol Med 44:169–179
Reid MB, Stokic DS, Koch SM, Khawli FA, Leis AA (1994) N-Acetylcysteine inhibits muscle fatigue in humans. J Clin Invest 94:2468–2474
Ristow M, Schmeisser S (2011) Extending life span by increasing oxidative stress. Free Radic Biol Med 51:327–336
Sakellariou GK, Vasilaki A, Palomero J, Kayani A, Zibrik L, McArdle A, Jackson MJ (2013) Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid Redox Signal 18:603–621
Sandström ME, Zhang SJ, Silva JP, Reid MB, Westerblad H, Katz A (2006) Role of reactive oxygen species in contraction-mediated glucose transport in skeletal muscle. J Physiol 575:251–262
Shindoh C, DiMarco A, Thomas A, Manubay P, Supinski G (1990) Effect of N-acetylcysteine on diaphragm fatigue. J Appl Physiol 68:2107–2113
Smith MA, Reid MB (2006) Redox modulation of contractile function in respiratory and limb skeletal muscle. Respir Physiol Neurobiol 151:229–241
Spencer MK, Katz A (1991) Role of glycogen in control of glycolysis and IMP formation in human muscle during exercise. Am J Physiol 260:E859–E864
Spencer MK, Yan Z, Katz A (1992) Effect of low glycogen on carbohydrate and energy metabolism in human muscle during exercise. Am J Physiol 262:C975–C979
Stull JT, Kamm KE, Vandenboom R (2011) Myosin light chain kinase and the role of myosin light chain phosphorylation in skeletal muscle. Arch Biochem Biophys 510:120–128
Supinski GS, Stofan D, Ciufo R, DiMarco A (1997) N-Acetylcysteine administration alters the response to inspiratory loading in oxygen-supplemented rats. J Appl Physiol 82:1119–1125
Szeto HH (2006) Mitochondria-targeted peptide antioxidants: novel neuroprotective agents. AAPS J 8:E521–E531
Travaline JM, Sudarshan S, Roy BG, Cordova F, Leyenson V, Criner GJ (1997) Effect of N-acetylcysteine on human diaphragm strength and fatigability. Am J Respir Crit Care Med 156:1567–1571
van der PC, Edwards JN, Macdonald WA, Stephenson DG (2007) Mitochondrial superoxide production in skeletal muscle fibers of the rat and decreased fiber excitability. Am J Physiol Cell Physiol 292:C1353–C1360
Westerblad H, Allen DG (1993) The contribution of [Ca2+]i to the slowing of relaxation in fatigued single fibres from mouse skeletal muscle. J Physiol 468:729–740
Westerblad H, Allen DG (2011) Emerging roles of ROS/RNS in muscle function and fatigue. Antioxid Redox Signal 15:2487–2499
Westerblad H, Lännergren J, Allen DG (1997) Slowed relaxation in fatigued skeletal muscle fibers of Xenopus and mouse. Contribution of [Ca2+]i and cross-bridges. J Gen Physiol 109:385–399
Weyand PG, Sandell RF, Prime DN, Bundle MW (2010) The biological limits to running speed are imposed from the ground up. J Appl Physiol 108:950–961
Whitehead NP, Pham C, Gervasio OL, Allen DG (2008) N-Acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice. J Physiol 586:2003–2014
Whitehead NP, Yeung EW, Froehner SC, Allen DG (2010) Skeletal muscle NADPH oxidase is increased and triggers stretch-induced damage in the mdx mouse. PLoS One 5:e15354
Zhang SJ, Andersson DC, Sandstrom ME, Westerblad H, Katz A (2006) Cross-bridges account for only 20% of total ATP consumption during submaximal isometric contraction in mouse fast-twitch skeletal muscle. Am J Physiol Cell Physiol 291:C147–C154
Zhang SJ, Sandström ME, Aydin J, Westerblad H, Wieringa B, Katz A (2008) Activation of glucose transport and AMP-activated protein kinase during muscle contraction in adenylate kinase-1 knockout mice. Acta Physiol (Oxf) 192:413–420
Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH (2004) Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem 279:34682–34690
Acknowledgments
This research was supported by grants from the Swedish National Center for Sports Research and the Swedish Research Council and by funds from the Karolinska Institutet. A. Hernández was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (no. IF32AR057619).
Conflict of interest
No conflict of interest, financial or the like, is declared by the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Katz, A., Hernández, A., Caballero, D.M.R. et al. Effects of N-acetylcysteine on isolated mouse skeletal muscle: contractile properties, temperature dependence, and metabolism. Pflugers Arch - Eur J Physiol 466, 577–585 (2014). https://doi.org/10.1007/s00424-013-1331-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-013-1331-z