Abstract
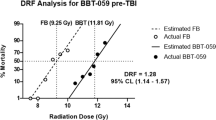
The histone deacetylase inhibitor (HDAC), phenylbutyrate (PB), is a novel anti-tumor agent. Studies have demonstrated that HDAC inhibitors can suppress cutaneous radiation syndrome and stimulate hematopoiesis. The objective of this study was to test the ability of PB treatment to protect against acute gamma-radiation-induced lethality in the DBA/2 mouse model. A 30-day radiation lethality study was used to assess radioprotective capability of PB. Mechanisms were evaluated using western blots, flow cytometry, and the single-cell gel electrophoresis assay. Western blot studies showed that PB treatment acetylated histones in vivo. For radiation protection studies, prophylactic administration of PB (24 h preradiation; 1–50 mg/kg) provided radioprotection against gamma radiation (8–9.5 Gy) and PB demonstrated a DRF of 1.31 (P = 0.001; 95% confidence interval: 1.27, 1.36). When PB (10 mg/kg) was administered post-radiation (4 h), it also provided significant radioprotection at 8.0 Gy radiation (P = 0.022). PB treatment before radiation was associated with significant elevations in neutrophils and platelets following radiation. Results from single-cell gel electrophoresis of peripheral blood leukocytes demonstrated that PB treatment before radiation can attenuate DNA damage and inhibit radiation-induced apoptosis. These results indicate that an HDAC inhibitor like PB has potential as a radiation protector and that mechanisms of action include attenuation of DNA damage and inhibition of apoptosis.











Similar content being viewed by others
Abbreviations
- PB:
-
Phenylbutyrate
- HDAC:
-
Histone deacetylase inhibitor
References
Brown SL et al (2008) Histone deacetylase inhibitors protect against and mitigate the lethality of total-body irradiation in mice. Radiat Res 169(4):474–478
Carducci MA et al (1997) Phenylbutyrate (PB) for refractory solid tumors: phase I clinical and pharmacological evaluation of intravenous and oral PB. Anticancer Res 17:3972–3973
Chung YL, Wang AJ, Yao LF (2004) Antitumor histone deacetylase inhibitors suppress cutaneous radiation syndrome: implications for increasing therapeutic gain in cancer radiotherapy. Mol Cancer Ther 3(3):317–325
Chung YL, Lee MY, Pui NN (2009) Epigenetic therapy using the histone deacetylase inhibitor for increasing therapeutic gain in oral cancer: prevention of radiation-induced oral mucositis and inhibition of chemical-induced oral carcinogenesis. Carcinogenesis 30(8):1387–1397
Davis T et al (2000) Histone deacetylase inhibitors decrease proliferation and modulate cell cycle gene expression in normal mammary epithelial cells. Clin Cancer Res 6:4334–4342
DiGiuseppe JA et al (1999) Phenylbutyrate-induced G1 arrest and apoptosis in myeloid leukemia cells: structure-function analysis. Leukemia Aug 13(8):1243–1253
Dover GJ, Brusilow S, Charache S (1994) Induction of fetal hemoglobin production in subjects with sickle cell anemia by oral sodium phenylbutyrate. Blood 84(1):339–343
Ferrandina G et al (2000) Growth inhibitory effects and radiosensitization induced by fatty aromatic acids on human cervical cancer cells. Oncol Res 12(9–10):429–440
Fibach E et al (1993) Enhanced fetal hemoglobin production by phenylacetate and 4-phenylbutyrate in erythroid precursors derived from normal donors and patients with sickle cell anemia and beta-thalassemia. Blood 82(7):2203–2209
Gore SD et al (1997) Clinical development of sodium phenylbutyrate (SPB) as a putative differentiating agent in myeloid malignancies. Anticancer Res 17:3981–3982
Gore SD et al (2002) Impact of prolonged infusions of the putative differentiating agent sodium phenylbutyrate on myelodysplastic syndromes and acute myeloid leukemia. Clin Cancer Res 8(4):963–970
Hafer N, Maidment BW, Hatchett RJ (2010) The NIAID radiation countermeasures program business model. Biosecurity Bioterrorism 8(4):357–363
Han S, Wada RK, Sidell N (2001) Differentiation of human neuroblastoma by phenylacetate is mediated by peroxisome proliferator-activated receptor gamma. Cancer Res 61:3998–4002
Haydont V et al (2007) Pravastatin inhibits the Rho/CCN2/extracellular matrix cascade in human fibrosis explants and improves radiation-induced intestinal fibrosis in rats. Clin Cancer Res 13(18 Pt 1):5331–5334
Hudgins WR, Fibach E, Safaya S, Rieder RF, Miller AC, Samid D (1996) Transcriptional upregulation of gamma-globin by phenylbutyrate and analogous aromatic fatty acids. Biochem Pharmacol 52(8):1227–1233
Jung M (2001) Inhibitors of histone deacetylase as new anticancer agents. Curr Med Chem 8:1505–1511
Katzung BG (2009) Basic and clinical pharmacology, 11th edn. McGraw-Hill, NY
Kim GD, Choi YH, Dimtchev A, Jeong SJ, Dritschilo A, Jung M (1999) Sensing of ionizing radiation-induced DNA damage by ATM through interaction with histone deacetylase. J Biol Chem 274:31127–31130
Końca K, Lankoff A, Banasik A, Lisowska H, Kuszewski T, Góźdź S, Koza Z, Wojcik A (2003) A cross platform public domain PC-image analysis program for the comet assay. Mutat Res 10:15–20
Liu L, Hudgins WR, Miller AC, Chen C, Samid D (1995) Transcriptional upregulation of TNF-alpha by phenylbutyrate. Cytokine 7(5):449–456
Lopez CA, Feng FY, Herman JM, Nyati MK, Lawrence TS, Ljungman M (2007) Phenylbutyrate sensitizes human glioblastoma cells lacking wild-type p53 function to ionizing radiation. Int J Radiat Oncol Biol Phys 69(1):214–220
Massague J, Blain SW, Lo RS (2001) TGFh signaling in growth control, cancer, and heritable disorders. Cell 103:295–309
Miller AC, Samid D (1997) Modification of radiation response based on the use of inhibitors of the mevalonate pathway of cholesterol synthesis. Adv Exp Med Biol 400B:825–830
Miller AC, Whittaker T, Thibault A, Samid D (1997) Modulation of radiation response of human tumor cells by the differentiation inducers, phenylacetate and phenylbutyrate. Int J Radiat Biol 72(2):211–218
Miller AC, Whittaker T, Page N (2001) Suppression of depleted uranium-induced neoplastic transformation of human cells by the phenyl fatty acid, phenyl acetate: chemoprevention by targeting the p21RAS protein pathway. Radiat Res 155(1 Pt 2):163–170
Nübel T, Damrot J, Roos W, Kaina B, Fritz G (2006) Lovastatin protects human endothelial cells from killing by ionizing radiation without impairing induction and repair of DNA double-strand breaks. Clin Cancer Res 2(3 Pt 1):933–939
Pace BS et al (2002) Short-chain fatty acid derivatives induce fetal globin expression and erythropoiesis in vivo. Blood 100(13):4640–4648
Paoluzzi L, Figg WD (2004) Histone deacetylase inhibitors are potent radiation protectants. Cancer Biol Ther 3(7):612–613
Prasanna P, Thibault A, Liu L, Samid D (1996) Lipid metabolism as a target for brain cancer therapy: synergistic activity of lovastatin and sodium phenylacetate against human glioma cells. J Neurochem 66:710–716
Resar LM, Segal JB, Fitzpatrick LK, Friedmann A, Brusilow SW, Dover GJ (2002) Induction of fetal hemoglobin synthesis in children with sickle cell anemia on low-dose oral sodium phenylbutyrate therapy. J Pediatr Hematol Oncol 9:737–741
Samid D, Shack S, Sherman LT (1992) Phenylacetate: a novel nontoxic inducer of tumor cell differentiation. Cancer Res 52:1988–1992
Samid D, Shack S, Myers CE (1993) Selective growth arrest and phenotypic reversion of prostate cancer cells in vitro by nontoxic pharmacological concentrations of phenylacetate. J Clin Investig 91:2288–2295
Samid D et al (1994) Selective activity of phenylacetate against malignant gliomas: resemblance to fetal brain damage in phenylketonuria. Cancer Res 54:891–895
Samid D, Hudgins WR, Shack S, Liu L, Prasanna P, Myers CE (1997) Phenylacetate and phenylbutyrate as novel, nontoxic differentiation inducers. Adv Exp Med Biol 400A:501–505
Samid D, Wells M, Greene ME, Shen W, Palmer CN, Thibault A (2000) Peroxisome proliferator-activated receptor gamma as a novel target in cancer therapy: binding and activation by an aromatic fatty acid with clinical antitumor activity. Clin Cancer Res 6:933–941
Seed TM (2002) New strategies for the prevention of radiation injury. J Radiat Res 43:S239–S244
Seed TM (2005) Radiation protectants: current status and future prospects. Health Phys 89(5):531–545
Singh VK, Brown DS, Kao TC (2010) Alpha-tocopherol succinate protects mice from gamma-radiation by induction of granulocyte-colony stimulating factor. Int J Radiat Biol 86(1):12–21
Thibault A, Figg WD, Samid D (1996) A Phase I study of the differentiating agent phenylbutyrate in patients with cancer. In: Proceedings annual meeting American Society Clinical Oncology 15:A1539
Wang J, Boerma M, Fu Q, Kulkarni A, Fink LM, Hauer-Jensen M (2007) Simvastatin ameliorates radiation enteropathy development after localized, fractionated irradiation by a protein C-independent mechanism. Int J Radiat Oncol Biol Phys 68(5):1483–1490
Weiss JF et al (1997) Modification of radiation-induced gastrointestinal and hematopoietic injury in mice by combinations of agents: effects of indomethacin and caffeine. Adv Exp Med Biol 400B:865–872
Whitnall MH, Inal CE, Jackson WE, Miner VL, Villmar V, Seed TM (2001) In vivo radioprotection against gamma-irradiation with 5-androstendiol: stimulation of the innate immune system. Radiat Res 156:283–293
Wieser R (2002) The transforming growth factor-h signaling pathway in tumorigenesis. Curr Opin Oncol 13:70–77
Wojewódzka M, Kruszewski M, Buraczewska I, Xu W, Massuda E, Zhang J, Szumiel I (2007) Sirtuin inhibition increases the rate of non-homologous end-joining of DNA double strand breaks. Acta Biochimie Polish 54(1):63–69
Záskodová D, Rezácová M, Vávrová J, Vokurková D, Tichy A (2006) Effect of valproic acid, a histone deacetylase inhibitor, on cell death and molecular changes caused by low-dose irradiation. Ann NY Acad Sci 1091:385–398
Acknowledgments
The authors are grateful to Dr. Christopher Lissner and Dr. John Gilstad for their encouragement and support of this work. The assistance of the AFRRI Veterinary Science Department was invaluable to the project. The authors would also like to thank the US Defense Threat Reduction Agency for their continued grant support (G1B2BJ and G1B2EM).
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miller, A.C., Cohen, S., Stewart, M. et al. Radioprotection by the histone deacetylase inhibitor phenylbutyrate. Radiat Environ Biophys 50, 585–596 (2011). https://doi.org/10.1007/s00411-011-0384-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-011-0384-7