Abstract
Introduction
Repetitive minor amputations carry the concomitant risks of multiple surgical procedures, major amputations have physical and economical major drawbacks. The aim of this study was to evaluate whether there is a distinct number of minor amputations predicting a major amputation in the same leg and to determine risk factors for major amputation in multiple minor amputations.
Materials and methods
A retrospective chart review including 429 patients with 534 index minor amputations between 07/1984 and 06/2019 was conducted. Patient demographics and clinical data including number and level of re-amputations were extracted from medical records and statistically analyzed.
Results
290 legs (54.3%) had one or multiple re-amputations after index minor amputation. 89 (16.7%) legs needed major amputation during follow up. Major amputation was performed at a mean of 32.5 (range 0 – 275.2) months after index minor amputation. No particular re-amputation demonstrated statistically significant elevated odds ratio (a.) to be a major amputation compared to the preceding amputation and (b.) to lead to a major amputation at any point during follow up. Stepwise multivariate Cox regression analysis revealed minor re-amputation within 90 days (HR 3.8, 95% CI 2.0-7.3, p <0.001) as the only risk factor for major amputation if at least one re-amputation had to be performed.
Conclusions
There is no distinct number of prior minor amputations in one leg that would justify a major amputation on its own. If a re-amputation has to be done, the timepoint needs to be considered as re-amputations within 90 days carry a fourfold risk for major amputation.
Level of evidence
Retrospective comparative study (Level III).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Minor foot amputations are common surgical interventions and feared consequences of complications in diabetic patients, patients with foot osteomyelitis, and patients suffering from peripheral arterial disease (PAD) [1,2,3,4,5,6,7,8,9,10,11,12]. Permanent disease (i.e. uncontrolled diabetes), or progression of atherosclerosis with consequent limb ischemia, often lead to more than one minor amputation [13,14,15,16,17]. A certain amount of these patients needs major amputation once the underlying conditions of the feet are not manageable anymore. In these multimorbid patients, each surgical intervention carries a substantial risk of perioperative morbidity, and therefore should be avoided or limited to a minimum, which in summary would favor an early major amputation [18,19,20,21,22]. However, major amputations lead to an increased oxygen consumption and cardiac effort, change the mobility level of the patient and necessitate auxiliary means [23,24,25,26,27,28].
Currently, the decision on whether to perform multiple minor amputations or an early major amputation strongly varies between countries and surgeons, but there is a trend in favor of performing multiple minor amputations [29, 30]. Consensus exists only about the importance of vascular perfusion when determining the amputation level [31,32,33]. Therefore, the decision to perform either multiple minor or one major amputation remains up to the surgeon’s expertise. Health instruments guiding the surgeon`s suggestion are missing [34].
Thus, the primary goal of this study was to investigate whether there is a threshold number of minor amputations that result in a major amputation. Secondary goal of the study was to identify risk factors for primary, and each other minor amputation, and to determine risk factors for major amputation in case of ≥ 2 minor amputations performed at the same foot.
Materials and methods
We conducted a retrospective chart review of prospectively collected data of all consecutive minor lower extremity amputations performed at our institution, which is specialized in the treatment of diabetic and dysvascular patients—between July 1st 1984 and November 30th, 2018. Inclusion criteria were first time minor amputation of the affected lower extremity, a possible passive follow up of at least one year and an active follow up of at least 90 days after index minor amputation. We refrained to exclude patients who died within 90 days after the index minor amputation and received major amputation prior to death. Amputations were considered “minor” until and including the Syme level and “major” starting with the transtibial level or more proximal. Exclusion criteria were prior amputation procedures at the same foot, amputations due to trauma, and malformations or tumor. A flowchart containing inclusion and exclusion details is given in Fig. 1. This study was approved by the local research ethics committee (BASEC number 2016–000387). Informed consent was obtained appropriate to local research ethics committee regulation.
Patient demographics and clinical data were derived from the institutional electronic medical records and are shown in Table 1. All amputations were performed by or under the direct supervision of one of two certified attending orthopedic surgeons (MCB or TB) with more than 15 years of surgical experience. When Osteomyelitis was clinically suspected (wound persisting > 3 months, bone visible within open wound, positive probe to bone test), it was confirmed by a combination of radiographs and MRI or nuclear medicine techniques (the latter when MRI was impossible due to implants such as pacemakers). The extent of osteomyelitis and thereby the amount of bone to be resected was determined by the amount of loss of focal signal in T1 sequences [35]. Information collected included patient demographic data, medical history and surgical details of amputations. Peripheral arterial disease was graded according to the Fontaine classification in stages 1 to 4 by angiologists [36]. The Fontaine classification is based on the clinical presentation of peripheral arterial disease (PAD) and contains four stages: stage one is asymptomatic PAD, stage two demonstrates mild claudication (IIA: claudication at a distance > 200 m, IIB: claudication at a distance < 200 m), stage three pain at rest while stage four demonstrates necrosis and/or gangrene [36]. Follow-up visits were scheduled according to a standardized scheme: the first visit was performed one week after hospital discharge. Further visits were scheduled every 7–10 days as long as the surgical wound or plantar ulcers were not healed and until transfer from the postoperative off-loading device (e.g. therapy shoe or cast) to a definitive foot protecting device (ranging from orthopedic insoles to orthopedic shoewear) had been realized. Upon verification of wound and/or ulcer healing, the next visit was scheduled 4–6 six weeks later. Given there were no skin lesions at this 4–6 six weeks visit, the next visits were scheduled every three months. Visits occurred either in our hospital`s outpatient clinic or in our wound care nursery.
Statistical analysis was conducted with SPSS statistical software (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.) Differences between groups were checked using students t-test for continuous demographic variables and using Chi-square test categorical demographic variables. The association between the consecutive number of surgery and the extent of the respective amputation was investigated using Chi-square tests. Odds ratios were calculated to compare the percentage of major amputations in rising numbers of re-amputations and to compare the percentage of major amputation during the further follow-up depending on the number of necessary minor re-amputations.
Kaplan–Meier survival estimates were calculated for major amputation free survival. The time of major amputation was selected as the primary endpoint. Following events were used as censor dates: death without major amputation; the last confirmed date without major amputation if the patient was lost to follow-up; date of data collection for this study, if no major amputation was done until this moment. Additionally, separate survival curves were plotted for different potential risk factors. Different survival curves were compared with the log-rank test.
In addition, the Hazard Ratio for major amputation was calculated for different patient groups using stepwise multivariate Cox regression analysis. Significance level was set to 0.05 for p-levels.
Results
Patient characteristics
There were 429 patients—319 men and 110 women – with 534 legs included. Mean follow-up was 49 (range 0 – 279) months. 91 (21.2%) patients died during follow-up at a mean of 63 (range 3 to 209) months after index minor amputation. Four patients died after having major amputation within 90 days.
Minor and major re-amputations
In all 534 legs of our study population, a minor amputation was performed as the index procedure. 290 (54.3%) legs had one or more re-amputation. 89 (16.7%) legs had to undergo major amputation during follow up. Of the 89 major amputations, 47 major amputations were performed as the first revision after index minor amputations. 42 major amputations were performed after one or several minor re-amputations. Major amputation was performed after a mean of 32.5 (range 0 – 275) months after index minor amputation.
129/290 (44.5%) re-amputation cases needed their re-amputation within the first 90 days after index minor amputation; 98 cases (33.8%) needed a minor re-amputation, 20 cases (6.9%) a major amputation and 11 (3.8%) cases both a minor and a major amputation.
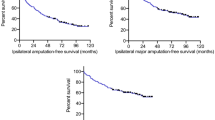
Major amputation free survival rate was 89.4% after 1 year (SD 1.4%; Number at risk 405), 83.5% after 5 years (SD 1.9%, Number at risk 132) and 75.3% after 10 years (SD 3.4%, Number at risk 39) (Fig. 2). The median major amputation free survival time was 16.1 years (95%CI 15.2 – 16.9 years).
Table 2 summarizes the results stratified by comorbidities diabetes mellitus and PAD.
Influence of timepoint and number of minor re-amputations on major amputation risk
Most of the patients had one or two re-amputations (Table 3). We first compared re-amputations pairwise to determine the odds ratio of the next re-amputation to be a major amputation: there was no re-amputation step that had a significantly elevated odds ratio to be a major amputation compared to the preceding one. Subsequently we calculated the odds ratio for each re-amputation step to suffer a major amputation at any point during further follow up. Again, all calculated odds ratios were insignificant (Table 3).
In cases without major amputation within the first 90 days after index amputation, patients without any re-amputation procedure within 90 days had a significant better major amputation free survival than patients that needed an early minor re-amputation (Log-Rank Test p = 0.04) (Fig. 3).
Kaplan–Meier survival estimates were performed for major amputation free survival, separated for each operation (index minor amputation, revisions 1 to 4; revision 5–7 were left out due to the limited number of cases). Revisions 1 to 4 demonstrated no statistical significance (Fig. 4, Log-Rank Tests given in Figure caption).
Risk factors for major amputation
Stepwise multivariate Cox regression analysis revealed peripheral arterial disease stage 3–4 (HR 2.3, 95% CI 1.5—3.6, p < 0.001) and minor re-amputation within 90 days (HR 1.6, 95% CI 1.0–2.6, p = 0.032) as risk factors for major amputation. Osteomyelitis was negatively associated with major amputation (Cox regression: HR 0.6, 95% CI 0.4 – 0.9, p = 0.012).
Investigating only those patients with multiple minor amputations, stepwise multivariate Cox regression analysis revealed minor amputation within 90 days after index amputation as the only significant risk factor for major amputation (HR 3.8, 95% CI 2.0–7.3, p < 0.001).
Kaplan–Meier survival curves were estimated comparing major amputation free survival in patients with PAD stages 1–2 versus 3–4 (Fig. 5), and combining presence of diabetes and PAD (Fig. 6). Results of the Cox regression analysis were confirmed.
Discussion
The primary goal of this study was to investigate whether there is a threshold of minor amputations, that increases the probability of a future major amputation. No minor amputation step had statistically significant increased odds to be followed by a major amputation as the next step. Further, no minor amputation step demonstrated increased odds to have a major amputation as any subsequent amputation step. Hence, we could not identify a clear threshold of minor amputations that constantly leads to a major amputation. The only variable that was associated with major amputation in case of multiple minor amputations was minor revision surgery within 90 days of the index minor amputation.
Amputations are a feared consequence of complications in long-term diabetes mellitus and PAD [1,2,3,4,5]. Many of the concerned patients have to undergo more than one minor or even a major amputation [13,14,15,16,17]. Due to the numerous and significant comorbidities of these patients, each amputation contains a major risk for severe cardiac, renal, or other complications even if minor amputations can be performed in regional anaesthesia [18,19,20,21,22]. To date, there is no evidence leaning towards multiple minor versus one major amputation. Our study supports those who advocate multiple minor amputations. Neither PAD nor Diabetes were identified as independent risk factors for major amputation in case of multiple minor amputations. This allows surgeons to apply our results on both patient collectives.
PAD is a known risk factor for lower extremity amputations. Major amputation has declined over the last decade but is still performed for 7% of patients with peripheral artery disease over the course of their lifetime [37,38,39,40,41]. Nerone et al. compared patients with at least 1 subsequent minor amputation with patients with at least 1 subsequent major amputation after an initial minor lower extremity amputation in diabetic patients [42]. He found that the prevalence of major amputation after initial minor pedal amputation was statistically significantly associated with the presence of peripheral arterial disease. Gurney et al. found in a national cohort of people with diabetes that peripheral vascular disease conferred the greatest independent risk for major amputation (adjusted HR of 12.72) of all tested comorbid conditions [43]. We were able to confirm severe PAD stages as a risk factor for major amputation after initial minor amputation and were able to show that this is not only true for diabetic patients but for overall patients undergoing minor amputation. However, analyzing patients with multiple minor amputations only, PAD stages 3 and 4 lost their status as a risk factor for major amputation. We consider this finding logical and assume that it has been partially influenced by a bias of indication. In severe PAD stages, loss of tissue can make limb preservation impossible and therefore exclude the possibility of multiple minor amputations.
Griffin described a significant correlation between patients who did not have diabetes and future limb loss after toe amputation [44]. We could not confirm this effect, but diabetes had no impact on major amputation free survival in our study. This is consistent with the results of Sheahan et al., who reported that diabetes does not have an impact on limb salvage in their series of 920 episodes with minor amputations [45].
The seemingly protective effect of osteomyelitis on major amputation free survival is an interesting observation of our study. We attribute this finding to better vascular patency and to a lengthy antibiotic treatment in most of the osteomyelitis cases. Our results demonstrate that Osteomyelitis in diabetic patients with PAD stage 1–2 seems to be more benign than in diabetic patients with PAD stage 3–4 and gangrene.
Minor amputation within 90 days after index amputation was the single independent risk factor we could identify for major amputation in case of multiple minor amputations. Interestingly, the proportion of patients that needed revision minor amputation within 90 days was similar among patients with Diabetes (19%) and PAD (18%). Patients without Diabetes were less affected by early revision minor amputation (16%). We assume that presence of microangiopathy played a distinct role in early revision. While we did not investigate presence of microangiopathy this remains an assumption. As surgeons could be tempted to perform major amputation in this "revision within 90 days" scenario, the authors want to emphasize the importance of repeated vascular work-up in those cases before any irreversible decision is made.
In the authors opinion, the synthesis of the results of the present study is that multiple minor amputations at the same foot are worthwhile to be considered, depending on key comorbidities (first and foremost PAD stages 3 and 4) and on the chronological sequence after the index amputation.
Strengths of the study are the length of follow-up and the comparatively high number of patients. Also, the study reflects the expertise of two surgeons with over 15 years of experience each. However, the study has several limitations: First, it is a retrospective study design of prospectively collected data. Both collection and treatment bias would be conceivable. Second, despite the comparatively high number of patients, the numbers in individual groups were smaller so other influencing factors may not have been identified as significant. Third, the vascular evaluation before minor amputation, from the historical point of view, differs among the patients at the begin of the study and nowadays. Historically the complete vascular assessment was performed only in patients with PAD. In last 10 years vascular assessment was part of investigation before the minor amputation (ABI, or ultrasound examination followed by CTA, or angiography if the vascular perfusion was not adequate).
The authors conclude that there is no distinct number of prior minor amputations in one leg that would justify a major amputation on its own. The only variable that is associated with major amputation in case of multiple minor amputations is a minor amputation within 90 days after index amputation. It carries a fourfold risk of major amputation. The time point of re-amputation necessity must be considered when choosing the appropriate amputation level.
References
Boyko EJ, Seelig AD, Ahroni JH (2018) Limb- and Person-Level Risk Factors for Lower-Limb Amputation in the Prospective Seattle Diabetic Foot Study. Diabetes Care 41(4):891–898. https://doi.org/10.2337/dc17-2210
Hospital discharge rates for nontraumatic lower extremity amputation by diabetes status--United States, 1997 (2001). MMWR Morbidity and mortality weekly report 50 (43):954–958
Moxey PW, Gogalniceanu P, Hinchliffe RJ, Loftus IM, Jones KJ, Thompson MM, Holt PJ (2011) Lower extremity amputations–a review of global variability in incidence. Diabet Med 28(10):1144–1153. https://doi.org/10.1111/j.1464-5491.2011.03279.x
Dormandy J, Heeck L, Vig S (1999) Major amputations: clinical patterns and predictors. Semin Vasc Surg 12(2):154–161
Lai YJ, Hu HY, Lin CH, Lee ST, Kuo SC, Chou P (2015) Incidence and risk factors of lower extremity amputations in people with type 2 diabetes in Taiwan, 2001–2010. J Diabetes 7(2):260–267. https://doi.org/10.1111/1753-0407.12168
Waibel FWA, Klammer A, Götschi T, Uçkay I, Böni T, Berli MC (2019) Outcome After Surgical Treatment of Calcaneal Osteomyelitis. Foot Ankle Int 40(5):562–567. https://doi.org/10.1177/1071100718822978
Häller TV, Kaiser P, Kaiser D, Berli MC, Uçkay I, Waibel FWA (2020) Outcome of Ray Resection as Definitive Treatment in Forefoot Infection or Ischemia: A Cohort Study. J Foot Ankle Surg 59(1):27–30. https://doi.org/10.1053/j.jfas.2019.06.003
Kaiser P, Häller TV, Uçkay I, Kaiser D, Berli M, Böni T, Waibel F (2019) Revision After Total Transmetatarsal Amputation. J Foot Ankle Surg 58(6):1171–1176. https://doi.org/10.1053/j.jfas.2019.03.015
Huseynova K, Sutradhar R, Booth GL, Huang A, Ray JG (2018) Risk of contralateral lower limb amputation and death after initial lower limb amputation - a population-based study. Heliyon 4(10):e00836. https://doi.org/10.1016/j.heliyon.2018.e00836
Huang YY, Lin CW, Yang HM, Hung SY, Chen IW (2018) Survival and associated risk factors in patients with diabetes and amputations caused by infectious foot gangrene. J Foot Ankle Res 11:1. https://doi.org/10.1186/s13047-017-0243-0
Remes L, Isoaho R, Vahlberg T, Hiekkanen H, Korhonen K, Viitanen M, Rautava P (2008) Major lower extremity amputation in elderly patients with peripheral arterial disease: incidence and survival rates. Aging Clin Exp Res 20(5):385–393. https://doi.org/10.1007/bf03325142
Kelly DA, Pedersen S, Tosenovsky P, Sieunarine K (2017) Major Lower Limb Amputation: Outcomes are Improving. Ann Vasc Surg 45:29–34. https://doi.org/10.1016/j.avsg.2017.05.039
Vadiveloo T, Jeffcoate W, Donnan PT, Colhoun HC, McGurnaghan S, Wild S, McCrimmon R, Leese GP, Scottish Diabetes Research Network Epidemiology G (2018) Amputation-free survival in 17,353 people at high risk for foot ulceration in diabetes: a national observational study. Diabetologia 61(12):2590–2597. https://doi.org/10.1007/s00125-018-4723-y
Thorud JC, Jupiter DC, Lorenzana J, Nguyen TT, Shibuya N (2016) Reoperation and Reamputation After Transmetatarsal Amputation: A Systematic Review and Meta-Analysis. J Foot Ankle Surg 55(5):1007–1012. https://doi.org/10.1053/j.jfas.2016.05.011
Acar E, Kacira BK (2017) Predictors of Lower Extremity Amputation and Reamputation Associated With the Diabetic Foot. J Foot Ankle Surg 56(6):1218–1222. https://doi.org/10.1053/j.jfas.2017.06.004
Kono Y, Muder RR (2012) Identifying the incidence of and risk factors for reamputation among patients who underwent foot amputation. Ann Vasc Surg 26(8):1120–1126. https://doi.org/10.1016/j.avsg.2012.02.011
Chu YJ, Li XW, Wang PH, Xu J, Sun HJ, Ding M, Jiao J, Ji XY, Feng SH (2016) Clinical outcomes of toe amputation in patients with type 2 diabetes in Tianjin. China Int Wound J 13(2):175–181. https://doi.org/10.1111/iwj.12249
Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R (2008) Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil 89(3):422–429. https://doi.org/10.1016/j.apmr.2007.11.005
Inderbitzi R, Buettiker M, Enzler M (2003) The long-term mobility and mortality of patients with peripheral arterial disease following bilateral amputation. Eur J Vasc Endovasc Surg 26(1):59–64. https://doi.org/10.1053/ejvs.2002.1868
Robbins JM, Strauss G, Aron D, Long J, Kuba J, Kaplan Y (2008) Mortality rates and diabetic foot ulcers: is it time to communicate mortality risk to patients with diabetic foot ulceration? J Am Podiatr Med Assoc 98(6):489–493. https://doi.org/10.7547/0980489
Moulik PK, Mtonga R, Gill GV (2003) Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care 26(2):491–494. https://doi.org/10.2337/diacare.26.2.491
Stern JR, Wong CK, Yerovinkina M, Spindler SJ, See AS, Panjaki S, Loven SL, D’Andrea RF Jr, Nowygrod R (2017) A Meta-analysis of Long-term Mortality and Associated Risk Factors following Lower Extremity Amputation. Ann Vasc Surg 42:322–327. https://doi.org/10.1016/j.avsg.2016.12.015
Pinzur MS, Gottschalk F, Smith D, Shanfield S, de Andrade R, Osterman H, Roberts JR, Orlando-Crombleholme P, Larsen J, Rappazzini P et al (1993) Functional outcome of below-knee amputation in peripheral vascular insufficiency. A multicenter review. Clin Orthop Relat Res 286:247–249
Pinzur MS (1993) Gait analysis in peripheral vascular insufficiency through-knee amputation. J Rehabil Res Dev 30(4):388–392
Pinzur MS, Smith D, Tornow D, Meade K, Patwardhan A (1993) Gait analysis of dysvascular below-knee and contralateral through-knee bilateral amputees: a preliminary report. Orthopedics 16(8):875–879
Gjovaag T, Mirtaheri P, Starholm IM (2018) Carbohydrate and fat oxidation in persons with lower limb amputation during walking with different speeds. Prosthet Orthot Int 42(3):304–310. https://doi.org/10.1177/0309364617740237
Jarvis HL, Bennett AN, Twiste M, Phillip RD, Etherington J, Baker R (2017) Temporal Spatial and Metabolic Measures of Walking in Highly Functional Individuals With Lower Limb Amputations. Arch Phys Med Rehabil 98(7):1389–1399. https://doi.org/10.1016/j.apmr.2016.09.134
Darter BJ, Hawley CE, Armstrong AJ, Avellone L, Wehman P (2018) Factors Influencing Functional Outcomes and Return-to-Work After Amputation: A Review of the Literature. J Occup Rehabil 28(4):656–665. https://doi.org/10.1007/s10926-018-9757-y
Spoden M, Nimptsch U, Mansky T (2019) Amputation rates of the lower limb by amputation level - observational study using German national hospital discharge data from 2005 to 2015. BMC Health Serv Res 19(1):8. https://doi.org/10.1186/s12913-018-3759-5
Kröger K, Berg C, Santosa F, Malyar N, Reinecke H (2017) Lower Limb Amputation in Germany. Dtsch Arztebl Int 114(7):130–136. https://doi.org/10.3238/arztebl.2017.0130
Crawford F, Welch K, Andras A, Chappell FM (2016) Ankle brachial index for the diagnosis of lower limb peripheral arterial disease. Cochrane Database Syst Rev 9:CD010680. doi:https://doi.org/10.1002/14651858.CD010680.pub2
Poredos P, Rakovec S, Guzic-Salobir B (2005) Determination of amputation level in ischaemic limbs using tcPO2 measurement. Vasa 34(2):108–112. https://doi.org/10.1024/0301-1526.34.2.108
Burgess EM, Matsen FA 3rd, Wyss CR, Simmons CW (1982) Segmental transcutaneous measurements of PO2 in patients requiring below-the-knee amputation for peripheral vascular insufficiency. J Bone Joint Surg Am 64(3):378–382
Malay DS (2018) The Quest for a Health Measurement Instrument to Guide Selection of Amputation Level. J Foot Ankle Surg 57(5):859. https://doi.org/10.1053/j.jfas.2018.07.016
Donovan A, Schweitzer ME (2010) Use of MR imaging in diagnosing diabetes-related pedal osteomyelitis. Radiographics 30(3):723–736. https://doi.org/10.1148/rg.303095111
Fontaine R, Kim M, Kieny R (1954) Surgical treatment of peripheral circulation disorders. Helv Chir Acta 21(5–6):499–533
Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, Golzarian J, Gornik HL, Halperin JL, Jaff MR, Moneta GL, Olin JW, Stanley JC, White CJ, White JV, Zierler RE, Society for Cardiovascular A, Interventions, Society of Interventional R, Society for Vascular M, Society for Vascular S (2011) 2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 58(19):2020–2045. https://doi.org/10.1016/j.jacc.2011.08.023
Jones WS, Patel MR, Dai D, Subherwal S, Stafford J, Calhoun S, Peterson ED (2012) Temporal trends and geographic variation of lower-extremity amputation in patients with peripheral artery disease: results from U.S. Medicare 2000–2008. J Am Coll Cardiol 60 (21):2230–2236. doi:https://doi.org/10.1016/j.jacc.2012.08.983
Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, Group TIW (2007) Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 45 Suppl S:S5–67. doi:https://doi.org/10.1016/j.jvs.2006.12.037
Goodney PP, Beck AW, Nagle J, Welch HG, Zwolak RM (2009) National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg 50(1):54–60. https://doi.org/10.1016/j.jvs.2009.01.035
Gabel J, Jabo B, Patel S, Kiang S, Bianchi C, Chiriano J, Teruya T, Abou-Zamzam AM Jr, Vascular Quality I (2018) Analysis of Patients Undergoing Major Lower Extremity Amputation in the Vascular Quality Initiative. Ann Vasc Surg 46:75–82. https://doi.org/10.1016/j.avsg.2017.07.034
Nerone VS, Springer KD, Woodruff DM, Atway SA (2013) Reamputation after minor foot amputation in diabetic patients: risk factors leading to limb loss. J Foot Ankle Surg 52(2):184–187. https://doi.org/10.1053/j.jfas.2012.11.015
Gurney JK, Stanley J, York S, Rosenbaum D, Sarfati D (2018) Risk of lower limb amputation in a national prevalent cohort of patients with diabetes. Diabetologia 61(3):626–635. https://doi.org/10.1007/s00125-017-4488-8
Griffin KJ, Rashid TS, Bailey MA, Bird SA, Bridge K, Scott JD (2012) Toe amputation: a predictor of future limb loss? J Diabetes Complications 26(3):251–254. https://doi.org/10.1016/j.jdiacomp.2012.03.003
Sheahan MG, Hamdan AD, Veraldi JR, McArthur CS, Skillman JJ, Campbell DR, Scovell SD, Logerfo FW, Pomposelli FB Jr (2005) Lower extremity minor amputations: the roles of diabetes mellitus and timing of revascularization. J Vasc Surg 42(3):476–480. https://doi.org/10.1016/j.jvs.2005.05.003
Funding
Open Access funding provided by Universität Zürich. No funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Ethical approval
This study was approved by the local research ethics committee (BASEC number 2016–000387).
Informed consent
Informed consent was obtained appropriate to local research ethics committee regulation.
Location of work statement
The study was conducted at Balgrist University Hospital, Zurich, Switzerland.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Berli, M.C., Rancic, Z., Schöni, M. et al. Salami-Tactics: when is it time for a major cut after multiple minor amputations?. Arch Orthop Trauma Surg 143, 645–656 (2023). https://doi.org/10.1007/s00402-021-04106-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-021-04106-5