Abstract
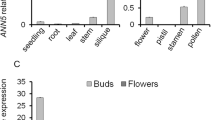
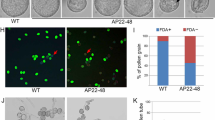
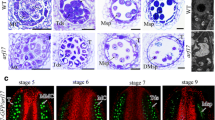
Microgametogenesis is a complex process that involves numerous well-coordinated cell activities, ending with the production of pollen grains. Pollen development has been studied at the cytological level in Arabidopsis and other plant species, where its temporal time course has been defined. However, the molecular mechanism underlying this process is still unclear, since a relative small number of genes and/or processes have been identified as essential for pollen development. We have designed a methodology to select candidate genes for functional analysis, based on transcriptomic data obtained from different stages of pollen development. From our analyses, we selected At2g22950 as a candidate gene; this gene encodes a protein belonging to the auto-regulated Ca2+-ATPase family, ACA7. Microarray data indicate that ACA7 is expressed exclusively in developing pollen grains, with the highest level of mRNA at the time of the second pollen mitosis. Our RT-PCR experiments showed that ACA7 mRNA is detected exclusively in developing flowers. Confocal microscopy experiments showed a plasma membrane localization for the recombinant GFP:ACA7 protein. We identified two different insertional mutant lines, aca7-1 and aca7-2; plants from both mutant lines displayed a normal vegetative development but showed large amounts of dead pollen grains in mature flowers assayed by Alexander’s staining. Histological analysis indicated that abnormalities are detected after the first pollen mitosis and we found a strong correlation between ACA7 mRNA accumulation and the severity of the phenotype. Our results indicate that ACA7 is a plasma membrane protein that has an important role during pollen development, possibly through regulation of Ca2+ homeostasis.





Similar content being viewed by others
References
Alexander MP (1969) Differential staining of aborted and nonaborted pollen. Stain Technol 44:117–122
Alonso JM, Stepanova AN, Leisse TJ et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301:653–657
Bonhomme S, Horlow C, Vezon D, de Laissardière S, Guyon A, Férault M, Marchand M, Bechtold N, Pelletier G (1998) T-DNA mediated disruption of essential gametophytic genes in Arabidopsis is unexpectedly rare and cannot be inferred from segregation distortion alone. Mol Gen Genet 260:444–452
Bonza MC, Morandini P, Luoni L, Geisler M, Palmgren MG, De Michelis MI (2000) At-ACA8 encodes a plasma membrane-localized calcium-ATPase of Arabidopsis with a calmodulin-binding domain at the N terminus. Plant Physiol 123:1495–1506
Boursiac Y, Harper JF (2007) The origin and function of calmodulin regulated Ca2+ pumps in plants. J Bioenerg Biomembr 39:409–414
Breeze E, Harrison E, McHattie S et al (2011) High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell 23:873–894
Brewbaker J, Kwack B (1963) The essential role of calcium ion in pollen germination and pollen tube growth. Am J Bot 50:859–865
Cannon SB, Mitra A, Baumgarten A, Young ND, May G (2004) The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol 4:10
Čapková V, Hrabětová E, Tupý J (1988) Protein synthesis in pollen tubes: preferential formation of new species independent of transcription. Sex Plant Reprod 1:150–155
Chehab EW, Kim S, Savchenko T, Kliebenstein D, Dehesh K, Braam J (2011) Intronic T-DNA insertion renders Arabidopsis opr3 a conditional jasmonic acid-producing mutant. Plant Physiol 156:770–778
Chen SH, Liao JP, Lup MZ, Kirchoff B (2008) Calcium distribution and function during anther development of Toreneiafournieri (Linderniaceae). Ann Bot Fennici 45:195–203
Costaglioli P, Joubès J, Garcia C et al (2005) Profiling candidate genes involved in wax biosynthesis in Arabidopsis thaliana by microarray analysis. BBA Mol Cell Biol L 1734:247–258
Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139:5–17
Dixon DP, Hawkins T, Hussey PJ, Edwards R (2009) Enzyme activities and subcellular localization of members of the Arabidopsis glutathione transferase superfamily. J Exp Bot 60:1207–1218
Funck D, Eckard S, Müller G (2010) Non-redundant functions of two proline dehydrogenase isoforms in Arabidopsis. BMC Plant Biol 10:70
George L, Romanowsky SM, Harper JF, Sharrock RA (2008) The ACA10 Ca2+-ATPase regulates adult vegetative development and inflorescence architecture in Arabidopsis. Plant Physiol 146:716–728
Gibeaut DM, Hulett J, Cramer GR, Seemann JR (1997) Maximal biomass of Arabidopsis thaliana using a simple, low-maintenance hydroponic method and favorable environmental conditions. Plant Physiol 115:317–319
Harper JF, Hong B, Hwang I, Guo HQ, Stoddard R, Huang JF, Palmgren MG, Sze H (1998) A novel calmodulin-regulated Ca2+-ATPase (ACA2) from Arabidopsis with an N-terminal autoinhibitory domain. J Biol Chem 273:1099–1106
Holmes-Davis R, Tanaka CK, Vensel WH, Hurkman WJ, McCormick S (2005) Proteome mapping of mature pollen of Arabidopsis thaliana. Proteomics 5:4864–4884
Honys D, Twell D (2004) Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biol 5:R85
Howden R, Park SK, Moore JM, Orme J, Grossniklaus U, Twell D (1998) Selection of T-DNA-tagged male and female gametophytic mutants by segregation distortion in Arabidopsis. Genetics 149:621–631
Johnson-Brousseau SA, McCormick S (2004) A compendium of methods useful for characterizing Arabidopsis pollen mutants and gametophytically-expressed genes. Plant J 39:761–775
Kabała K, Kłobus G (2005) Plant Ca2+-ATPases. Acta Physiol Plant 27:559–574
Karimi M, Inzé D, Depicker A (2002) Gateway(™) vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7:193–195
Lalanne E, Michaelidis C, Moore JM et al (2004) Analysis of transposon insertion mutants highlights the diversity of mechanisms underlying male progamic development in Arabidopsis. Genetics 167:1975–1986
León G, Holuigue L, Jordana X (2007) Mitochondrial complex II is essential for gametophyte development in Arabidopsis. Plant Physiol 143:1534–1546
Malho R, Read ND, Trewavas AJ, Pais MS (1995) Calcium channel activity during pollen tube growth and reorientation. Plant Cell 7:1173–1184
Mascarenhas NT, Bashe D, Eisenberg A, Willing RP, Xiao CM, Mascarenhas JP (1984) Messenger RNAs in corn pollen and protein synthesis during germination and pollen tube growth. Theor Appl Genet 68:323–326
McCormick S (2004) Control of male gametophyte development. Plant Cell 16:S142–S153
Nelson BK, Cai X, Nebenführ A (2007) A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J51:1126–1136
Noir S, Bräutigam A, Colby T, Schmidt J, Panstruga R (2005) A reference map of the Arabidopsis thaliana mature pollen proteome. Biochem Biophys Res Commun 337:1257–1266
Pierson ES, Miller DD, Callaham DA, van Aken J, Hackett G, Hepler PK (1996) Tip-localized calcium entry fluctuates during pollen tube growth. Dev Biol 174:160–173
Pina C, Pinto F, Feijó JA, Becker JD (2005) Gene family analysis of the Arabidopsis pollen transcriptome reveals biological implications for cell growth, division control and gene expression regulation. Plant Physiol 138:744–756
Regan SM, Moffatt BA (1990) Cytochemical analysis of pollen development in wild-type Arabidopsis and a male-sterile mutant. Plant Cell 2:877–889
Sanders PM, Bui AQ, Weterings K et al (1999) Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex Plant Reprod 11:297–322
Schiøtt M, Palmgreen M (2005) Two plant Ca2+ pumps expressed in stomatal guard cells show opposite expression patterns during cold stress. Physiol Plantarum 124:278–283
Schiøtt M, Romanowsky SM, Bækgaard L, Jakobsen MK, Palmgren MG, Harper JF (2004) A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proc Natl Acad Sci USA 101:9502–9507
Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Schölkopf B, Weigel D, Lohmann JU (2005) A gene expression map of Arabidopsis thaliana development. Nat Genet 37:501–506
Soler M, Serra O, Molinas M, Huguet G, Fluch S, Figueras M (2007) A genomic approach to suberin biosynthesis and cork differentiation. Plant Physiol 144:419–431
Suh MC, Samuels AL, Jetter R et al (2005) Cuticular lipid composition, surface structure and gene expression in Arabidopsis stem epidermis. Plant Physiol 139:1649–1665
Taylor LP, Hepler PK (1997) Pollen germination and tube growth. Annu Rev Plant Physiol Plant Mol Biol 48:461–491
Yamamoto Y, Nishimura M, Hara-Nishimura I, Noguchi T (2003) Behavior of vacuoles during microspore and pollen development in Arabidopsis thaliana. Plant Cell Physiol 44:1192–1201
Acknowledgments
The authors are greatly indebted to Ariel Orellana and Reinaldo Campos for their constant encouragement and support. This work was supported by FONDECYT grant number 11080037 from the Chilean Government and Universidad Andres Bello VRID UNAB-DI-23-10/R to GL. NL is supported by a CONICYT fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Toriyama.
Rights and permissions
About this article
Cite this article
Lucca, N., León, G. Arabidopsis ACA7, encoding a putative auto-regulated Ca2+-ATPase, is required for normal pollen development. Plant Cell Rep 31, 651–659 (2012). https://doi.org/10.1007/s00299-011-1182-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-011-1182-z