Abstract
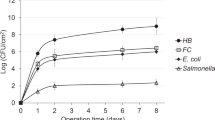
The zeta potentials of E. coli, GFP (green fluorescence protein)-labeled E. coli, Salmonella Newport, and Pseudomonas sp. in different states (nutrient-starved and dead) and grown in rich and minimal media were measured. Capillary electrophoresis experiments were conducted to measure the zeta potential of the different cells suspended in a drinking water sample. Salmonella Newport strain showed a lower zeta potential compared to E. coli, GFP-labeled E. coli, and Pseudomonas sp. Starved E. coli cells had a lower zeta potential compared to E. coli cells grown under rich media conditions. Salmonella Newport cells grown in minimal media also had a lower zeta potential compared to rich, starved, and dead cells. The different bacterial cell types exhibited differences in size as well. These results suggest that when bacterial cells are present in drinking water they can exhibit significant heterogeneity in the size and zeta potential, depending on their physiological state.
Similar content being viewed by others
References
Abramoff MD, Magelhaes PJ, Ram SJ (2004) Image processing with Image. ImageJ Biophoton Int 11(7):36–42
Buszewski B, Szumski M, Klodzinska E, et al. (2003) Separation of bacteria by capillary. J Sep Sci 26:1045–1049
Codony F, Morato J, Mas J (2005) Role of discontinuous chlorination on microbial production by drinking water biofilms. Water Res 39:1896–1906
Cowan MM, Van der Mei HC, Stokroos I, et al. (1992) Heterogeneity of surfaces of subgingival bacteria as detected by zeta potential measurements. J Dent Res 71(11):1803–1806
Endley S, Lu L, Vega E, et al. (2003) Male-specific coliphages as an additional fecal contamination indicator for screening fresh carrots. J Food Prot 66(1):88–93
Guan H, Schulze-Makuch D, Schaffer S, et al. (2003) The effect of critical pH on virus fate and transport in saturated porous medium. Groundwater 41:701–708
Hayashi H, Tsuneda S, Hirata A, et al. (2001) Soft particle analysis of bacterial cells and its interpretation of cell adhesion behaviors in terms of DLVO theory. Colloids Surf B Biointerf 2:149–157
Jones JF, Feick JD, Imoudu D, et al. (2003) Oriented adhesion of Escherichia coli to polystyrene particles. Appl Environ Microbiol 69(11): 6515–6519
Karniadakis G, Beskok A, Aluru N (2005) Miroflows and nanoflows: fundamentals and simulation. Springer, New York
Kilian HG, Gruler H, Bartkowiak D, et al. (2005) Stationary cell size distributions and mean protein chain length distributions of Archaea, Bacteria and Eukaryotes described with an increment model in terms of irreversible thermodynamics. Eur Phys J E 17:307–325
Kourkine IV, Mirjana RP, Davis E, et al. (2003) Detection of Escherichia coli O157:H7 bacteria by a combination of immunofluorescent staining and capillary electrophoresis. Electrophoresis 24:655–661
Lee JS, Ray RI, Lemieux EJ, Falster AU, et al. (2004) An evaluation of carbon steel corrosion under stagnant seawater conditions. Biofouling 20:237–247
Lewis PJ, Nwoguh CE, Barer MR, et al. (1994) Use of digitized video microscopy with a fluorogenic enzyme substrate to demonstrate cell- and compartment-specific gene expression in Salmonella enteritidis and Bacillus subtilis. Mol Microbiol 13(4):655–662
Lyman R, Longnecker M (2001) An introduction to statistical methods and data analysis. 5th ed. Wadsworth, Belmont, CA
Lytle DA, Rice EW, Clifford H, et al. (1999) Electrophoretic mobilities of Escherichia coli O157:H7 and wild-type Escherichia coli strains. Appl Environ Microbiol 65(7):3222–3225
Truesdail SE, Lukasik J, Farrah SR, et al. (1998) Analysis of bacterial deposition on metal (hydr)oxide-coated sand filter media. J. Colloid Interf Sci 203:369–378
van Merode AEJ, van der Mei HC, Busscher HJ, et al. (2006) Influence of culture heterogeneity in cell surface charge on adhesion and biofilm formation by Enterococcus faecalis. J Bacteriol 188(7):2421–2426
Wilson K, Walker J (2005) Principles and techniques of biochemistry and molecular biology. 6th ed. Cambridge University Press, Cambridge
Acknowledgments
This work was funded by NASA-AEMC program and the State of Texas Advanced Technology Programs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soni, K.A., Balasubramanian, A.K., Beskok, A. et al. Zeta Potential of Selected Bacteria in Drinking Water When Dead, Starved, or Exposed to Minimal and Rich Culture Media. Curr Microbiol 56, 93–97 (2008). https://doi.org/10.1007/s00284-007-9046-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-007-9046-z