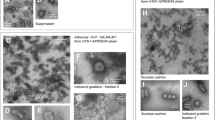
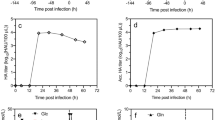
Abstract
Forced by major drawbacks of egg-based influenza virus production, several studies focused on the establishment and optimization of cell-based production systems. Among numerous possible host cell lines from duck, monkey, canine, chicken, mouse, and human origin, only a few will meet regulatory requirements, accomplish industrial standards, and result in high virus titers. From primary virus isolation up to large-scale manufacturing of human vaccines, however, the most logical choice seems to be the use of human cell lines. For this reason, we evaluated the recently established CAP cell line derived from human amniocytes for its potential in influenza virus production in suspension culture in small scale shaker flask and stirred tank bioreactor experiments. Different human and animal influenza viruses could be adapted to produce hemagglutination (HA) titers of at least 2.0 log10 HA units/100 μL without further process optimization. Adjusting trypsin activity as well as infection conditions (multiplicity of infection, infection medium) resulted in HA titers of up to 3.2 log10 HA units/100 μL and maximum cell-specific virus productivities of 6,400 virions/cell (for human influenza A/PR/8/34 as a reference). Surface membrane expression of sialyloligosaccharides as well as HA N-glycosylation patterns were characterized. Overall, experimental results clearly demonstrate the potential of CAP cells for achieving high virus yields for different influenza strains and the option to introduce a highly attractive fully characterized human cell line compliant with regulatory and industrial requirements as an alternative for influenza virus vaccine production.






Similar content being viewed by others
References
Aggarwal K, Jing F, Maranga L, Liu J (2011) Bioprocess optimization for cell culture based influenza vaccine production. Vaccine 29(17):3320–3328. doi:10.1016/j.vaccine.2011.01.081
Chu C, Lugovtsev V, Golding H, Betenbaugh M, Shiloach J (2009) Conversion of MDCK cell line to suspension culture by transfecting with human siat7e gene and its application for influenza virus production. Proc Natl Acad Sci U S A 106(35):14802–14807. doi:10.1073/pnas.0905912106
Chu C, Lugovtsev V, Lewis A, Betenbaugh M, Shiloach J (2010) Production and antigenic properties of influenza virus from suspension MDCK-siat7e cells in a bench-scale bioreactor. Vaccine 28(44):7193–7201. doi:10.1016/j.vaccine.2010.08.059
Fallaux FJ, Bout A, van der Velde I, van den Wollenberg DJ, Hehir KM, Keegan J, Auger C, Cramer SJ, van Ormondt H, van der Eb AJ, Valerio D, Hoeben RC (1998) New helper cells and matched early region 1-deleted adenovirus vectors prevent generation of replication-competent adenoviruses. Hum Gene Ther 9(13):1909–1917. doi:10.1089/hum.1998.9.13-1909
Gambaryan AS, Marinina VP, Tuzikov AB, Bovin NV, Rudneva IA, Sinitsyn BV, Shilov AA, Matrosovich MN (1998) Effects of host-dependent glycosylation of hemagglutinin on receptor-binding properties on H1N1 human influenza A virus grown in MDCK cells and in embryonated eggs. Virology 247(2):170–177
Genzel Y, Reichl U (2007) Vaccine production - state of the art and future needs in upstream processing. In: Pörtner R (ed) Methods in biotechnology: animal cell biotechnology—methods and protocols. Humana Press Inc., Totowa, pp 457–473
Genzel Y, Reichl U (2009) Continuous cell lines as a production system for influenza vaccines. Expert Rev Vaccines 8(12):1681–1692. doi:10.1586/erv.09.128
Genzel Y, Behrendt I, Konig S, Sann H, Reichl U (2004) Metabolism of MDCK cells during cell growth and influenza virus production in large-scale microcarrier culture. Vaccine 22(17–18):2202–2208. doi:10.1016/j.vaccine.2003.11.041
Genzel Y, Fischer M, Reichl U (2006) Serum-free influenza virus production avoiding washing steps and medium exchange in large-scale microcarrier culture. Vaccine 24(16):3261–3272. doi:10.1016/j.vaccine.2006.01.019
Genzel Y, Dietzsch C, Rapp E, Schwarzer J, Reichl U (2010) MDCK and Vero cells for influenza virus vaccine production: a one-to-one comparison up to lab-scale bioreactor cultivation. Appl Microbiol Biotechnol 88(2):461–475. doi:10.1007/s00253-010-2742-9
George M, Farooq M, Dang T, Cortes B, Liu J, Maranga L (2010) Production of cell culture (MDCK) derived live attenuated influenza vaccine (LAIV) in a fully disposable platform process. Biotechnol Bioeng 106(6):906–917. doi:10.1002/bit.22753
Govorkova EA, Kaverin NV, Gubareva LV, Meignier B, Webster RG (1995) Replication of influenza A viruses in a green monkey kidney continuous cell line (Vero). J Infect Dis 172(1):250–253
Graham FL, Smiley J, Russell WC, Nairn R (1977) Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol 36(1):59–74
Gregersen JP, Schmitt HJ, Trusheim H, Broker M (2011) Safety of MDCK cell culture-based influenza vaccines. Future Microbiol 6(2):143–152. doi:10.2217/fmb.10.161
Jordan I, Vos A, Beilfuss S, Neubert A, Breul S, Sandig V (2009) An avian cell line designed for production of highly attenuated viruses. Vaccine 27(5):748–756. doi:10.1016/j.vaccine.2008.11.066
Kalbfuss B, Knochlein A, Krober T, Reichl U (2008) Monitoring influenza virus content in vaccine production: precise assays for the quantitation of hemagglutination and neuraminidase activity. Biologicals 36(3):145–161
Kaverin NV, Webster RG (1995) Impairment of multicycle influenza virus growth in Vero (WHO) cells by loss of trypsin activity. J Virol 69(4):2700–2703
Le Ru A, Jacob D, Transfiguracion J, Ansorge S, Henry O, Kamen AA (2010) Scalable production of influenza virus in HEK-293 cells for efficient vaccine manufacturing. Vaccine 28(21):3661–3671. doi:10.1016/j.vaccine.2010.03.029
Lohr V, Rath A, Genzel Y, Jordan I, Sandig V, Reichl U (2009) New avian suspension cell lines provide production of influenza virus and MVA in serum-free media: studies on growth, metabolism and virus propagation. Vaccine 27(36):4975–4982. doi:10.1016/j.vaccine.2009.05.083
Lohr V, Genzel Y, Behrendt I, Scharfenberg K, Reichl U (2010) A new MDCK suspension line cultivated in a fully defined medium in stirred-tank and wave bioreactor. Vaccine 28(38):6256–6264. doi:10.1016/j.vaccine.2010.07.004
Mehtali M, Champion-Arnaud P, Leon A (2006) Process of manufacturing viral vaccines in suspension avian embryonic derived stem cell lines WO 2006108846 (A1)
Nishiyama K, Sugawara K, Nouchi T, Kawano N, Soejima K, Abe S, Mizokami H (2008) Purification and cDNA cloning of a novel protease inhibitor secreted into culture supernatant by MDCK cells. Biologicals 36(2):122–133. doi:10.1016/j.biologicals.2007.07.004
Paillet C, Forno G, Kratje R, Etcheverrigaray M (2009) Suspension-Vero cell cultures as a platform for viral vaccine production. Vaccine 27(46):6464–6467. doi:10.1016/j.vaccine.2009.06.020
Paillet C, Forno G, Soldano N, Kratje R, Etcheverrigaray M (2011) Statistical optimization of influenza H1N1 production from batch cultures of suspension Vero cells (sVero). Vaccine 29(41):7212–7217. doi:10.1016/j.vaccine.2011.07.016
Pau MG, Ophorst C, Koldijk MH, Schouten G, Mehtali M, Uytdehaag F (2001) The human cell line PER.C6 provides a new manufacturing system for the production of influenza vaccines. Vaccine 19(17–19):2716–2721
Petiot E, Jacob D, Lanthier S, Lohr V, Ansorge S, Kamen AA (2011) Metabolic and kinetic analyses of influenza production in perfusion HEK293 cell culture. BMC Biotechnol 11:84. doi:10.1186/1472-6750-11-84
Reading PC, Tate MD, Pickett DL, Brooks AG (2007) Glycosylation as a target for recognition of influenza viruses by the innate immune system. Adv Exp Med Biol 598:279–292. doi:10.1007/978-0-387-71767-8_20
Robertson JS, Bootman JS, Newman R, Oxford JS, Daniels RS, Webster RG, Schild GC (1987) Structural changes in the haemagglutinin which accompany egg adaptation of an influenza A(H1N1) virus. Virology 160(1):31–37
Robertson JS, Nicolson C, Harvey R, Johnson R, Major D, Guilfoyle K, Roseby S, Newman R, Collin R, Wallis C, Engelhardt OG, Wood JM, Le J, Manojkumar R, Pokorny BA, Silverman J, Devis R, Bucher D, Verity E, Agius C, Camuglia S, Ong C, Rockman S, Curtis A, Schoofs P, Zoueva O, Xie H, Li X, Lin Z, Ye Z, Chen LM, O’Neill E, Balish A, Lipatov AS, Guo Z, Isakova I, Davis CT, Rivailler P, Gustin KM, Belser JA, Maines TR, Tumpey TM, Xu X, Katz JM, Klimov A, Cox NJ, Donis RO (2011) The development of vaccine viruses against pandemic A(H1N1) influenza. Vaccine 29(9):1836–1843. doi:10.1016/j.vaccine.2010.12.044
Rödig JV, Rapp E, Djeljadini S, Lohr V, Genzel Y, Jordan I, Sandig V, Reichl U (2011a) Impact of influenza virus adaptation status on HA N-glycosylation patterns in cell culture-based vaccine production. J Carbohydr Chem 30(4–6):281–290. doi:10.1080/07328303.2011.604454
Rödig JV, Rapp E, Höper D, Genzel Y, Reichl U (2011b) Impact of host cell line adaptation on quasispecies composition and glycosylation of influenza A virus hemagglutinin. PLoS One 6(12):e27989. doi:10.1371/journal.pone.0027989
Sandig V, Jordan I (2005) Immortalized avian cell lines for virus production. WO2005/042728A2,
Schiedner G, Hertel S, Kochanek S (2000) Efficient transformation of primary human amniocytes by E1 functions of Ad5: generation of new cell lines for adenoviral vector production. Hum Gene Ther 11(15):2105–2116. doi:10.1089/104303400750001417
Schiedner G, Hertel S, Bialek C, Kewes H, Waschutza G, Volpers C (2008) Efficient and reproducible generation of high-expressing, stable human cell lines without need for antibiotic selection. BMC Biotechnol 8:13. doi:10.1186/1472-6750-8-13
Schwarzer J, Rapp E, Reichl U (2008) N-glycan analysis by CGE-LIF: profiling influenza A virus hemagglutinin N-glycosylation during vaccine production. Electrophoresis 29(20):4203–4214. doi:10.1002/elps.200800042
Schwarzer J, Rapp E, Hennig R, Genzel Y, Jordan I, Sandig V, Reichl U (2009) Glycan analysis in cell culture-based influenza vaccine production: influence of host cell line and virus strain on the glycosylation pattern of viral hemagglutinin. Vaccine 27(32):4325–4336. doi:10.1016/j.vaccine.2009.04.076
Seitz C, Frensing T, Hoper D, Kochs G, Reichl U (2010) High yields of influenza A virus in Madin-Darby canine kidney cells are promoted by an insufficient interferon-induced antiviral state. J Gen Virol 91(Pt 7):1754–1763. doi:10.1099/vir.0.020370-0
Seitz C, Isken B, Heynisch B, Rettkowski M, Frensing T, Reichl U (2012) Trypsin promotes efficient influenza vaccine production in MDCK cells by interfering with the antiviral host response. Appl Microbiol Biotechnol 93(2):601–611. doi:10.1007/s00253-011-3569-8
Shaw G, Morse S, Ararat M, Graham FL (2002) Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J Off Publ Fed Am Soc Exp Biol 16(8):869–871. doi:10.1096/fj.01-0995fje
Tobita K, Tanaka T, Hayase Y (1997) Rescue of a viral gene from Vero cells latently infected with influenza virus B/Lee/40. Virology 236(1):130–136
Tseng YF, Hu AY, Huang ML, Yeh WZ, Weng TC, Chen YS, Chong P, Lee MS (2011) Adaptation of high-growth influenza H5N1 vaccine virus in Vero cells: implications for pandemic preparedness. PLoS One 6(10):e24057. doi:10.1371/journal.pone.0024057
van Wielink R, Kant-Eenbergen HC, Harmsen MM, Martens DE, Wijffels RH, Coco-Martin JM (2011) Adaptation of a Madin-Darby canine kidney cell line to suspension growth in serum-free media and comparison of its ability to produce avian influenza virus to Vero and BHK21 cell lines. J Virol Methods 171(1):53–60. doi:10.1016/j.jviromet.2010.09.029
Vester D, Rapp E, Gade D, Genzel Y, Reichl U (2009) Quantitative analysis of cellular proteome alterations in human influenza A virus-infected mammalian cell lines. Proteomics. doi:10.1002/pmic.200800893
Vester D, Rapp E, Kluge S, Genzel Y, Reichl U (2010) Virus–host cell interactions in vaccine production cell lines infected with different human influenza A virus variants: a proteomic approach. J Proteomics 73(9):1656–1669. doi:10.1016/j.jprot.2010.04.006
Von Magnus P (1954) Incomplete forms of influenza virus. Adv Virus Res 2:59–79
Zhirnov OP, Vorobjeva IV, Saphonova OA, Poyarkov SV, Ovcharenko AV, Anhlan D, Malyshev NA (2009) Structural and evolutionary characteristics of HA, NA, NS and M genes of clinical influenza A/H3N2 viruses passaged in human and canine cells. J Clin Virol 45(4):322–333. doi:10.1016/j.jcv.2009.05.030
Acknowledgment
The authors would like to thank V. Lohr for critical proof reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Genzel, Y., Behrendt, I., Rödig, J. et al. CAP, a new human suspension cell line for influenza virus production. Appl Microbiol Biotechnol 97, 111–122 (2013). https://doi.org/10.1007/s00253-012-4238-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4238-2