Abstract
Purpose: New selective estrogen-receptor modulators for the treatment and prevention of osteoporosis, cardiovascular disease and breast cancer are currently the focus of intense research. (Deaminohydroxy)toremifene (Z-2-[4-(4-chloro-1,2-diphenyl-but-1-enyl)phenoxy]ethanol; FC-1271a) has been shown to prevent bone resorption in rats while having no or weak estrogen-like effects on the uterus, which makes it a good candidate drug for osteoporosis prevention. Our purpose here was to examine the pharmacokinetics of (deaminohydroxy)toremifene in humans included in two phase-I studies.
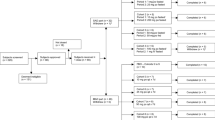
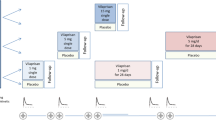
Methods: The first was a single-dose, dose-escalation study with 28 healthy male volunteers. Doses ranged from 10 mg to 800 mg. The second study was conducted during a 12-week period with 40 healthy, post-menopausal women, who received repeated oral doses of 25–200 mg. Standard pharmacokinetic parameters were assessed.
Results: In the single-dose study, time to reach peak concentration (tmax) ranged from 1.3 h to 4.0 h; peak concentration (Cmax) ranged from 15 ng/ml to 445 ng/ml; and the estimated terminal elimination half-life (mean ± SD; t1/2) was 24.8 ± 7.0 h. In the repeated-dose study, tmax ranged from 1.9 h to 2.6 h at 6 weeks and from 2.5 h to 2.9 h at 12 weeks. Cmax ranged from 295 ng/ml to 1043 ng/ml at 6 weeks and from 25 ng/ml to 1211 ng/ml at 12 weeks. The average t1/2 at all dose levels was 29.7 ± 1.5 h (overall mean ± SD). Strong linear correlations between the dose and Cmax and between the dose and the area under the curve were observed in both studies.
Conclusion: Our results indicate that (deaminohydroxy)toremifene has pharmacokinetics suitable for single daily dosing. The prophylactic use of this agent in women susceptible to development of osteoporosis, cardiovascular disease and breast cancer could, therefore, be tested using a once-daily dosing schedule similar to those of other hormone-replacement therapy regimens.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 12 July 1999 / Accepted in revised form: 18 May 2000
Rights and permissions
About this article
Cite this article
DeGregorio, M., Wurz, G., Taras, T. et al. Pharmacokinetics of (deaminohydroxy)toremifene in humans: a new, selective estrogen-receptor modulator. E J Clin Pharmacol 56, 469–475 (2000). https://doi.org/10.1007/s002280000176
Issue Date:
DOI: https://doi.org/10.1007/s002280000176