Abstract
Aim
The human UDP-glucuronosyltransferase which is genetically polymorphic catalyzes glucuronidations of various drugs. The interactions among UGT1A4, UGT1A6, UGT1A9, and UGT2B15 genetic polymorphisms, monohydroxylated derivative (MHD) of oxcarbazepine (OXC) plasma concentrations, and OXC monotherapeutic efficacy were explored in 124 Chinese patients with epilepsy receiving OXC monotherapy.
Method
MHD is the major active metabolite of OXC, and its plasma concentration was measured using high-performance liquid chromatography when patients reached their maintenance dose of OXC. Genomic DNA was extracted from whole blood and SNP genotyping performed using PCR followed by dideoxy chain termination sequencing. We followed the patients for at least 1 year to evaluate the OXC monotherapy efficacy. Patients were divided into two groups according to their therapeutic outcome: group 1, seizure free; group 2, not seizure free. The data were analyzed using T test, one-way analysis of variance (ANOVA), Kruskal-Wallis test, chi-square test, Fisher’s exact test, correlation analysis, and multivariate regression analysis.
Result
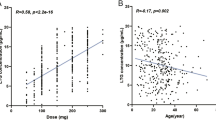
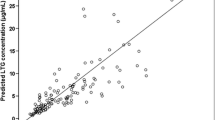
T test analysis showed that MHD plasma concentrations were significantly different between the two groups (p = 0.002). One-way ANOVA followed by Bonferroni post hoc testing of four candidate SNPs revealed that carriers of the UGT1A9 variant allele I399 C > T (TT 13.28 ± 7.44 mg/L, TC 16.41 ± 6.53 mg/L) had significantly lower MHD plasma concentrations and poorer seizure control than noncarriers (CC 22.24 ± 8.49 mg/L, p < 0.05).
Conclusion
In our study, we have demonstrated the effects of UGT1A9 genetic polymorphisms on MHD plasma concentrations and OXC therapeutic efficacy. Through MHD monitoring, we can predict OXC therapeutic efficacy, which may be useful for the personalization of OXC therapy in epileptic patients.



Similar content being viewed by others
References
Tecoma ES (1999) Oxcarbazepine. Epilepsia 40(Suppl 5):S37–S46
Armijo JA, Vega-Gil N, Shushtarian M, Adin J, Herranz JL (2005) 10-Hydroxycarbazepine serum concentration-to-oxcarbazepine dose ratio: influence of age and concomitant antiepileptic drugs. Ther Drug Monit 27:199–204
Shorvon S (2000) Oxcarbazepine: a review. Seizure 9:75–79
Stingl JC, Bartels H, Viviani R, Lehmann ML, Brockmöller J (2014) Relevance of UDP-glucuronosyltransferase polymorphisms for drug dosing: a quantitative systematic review. PHARMACOL THERAPEUT 141:92–116
Chang Y, Yang LY, Zhang MC, Liu SY (2014) Correlation of the UGT1A4 gene polymorphism with serum concentration and therapeutic efficacy of lamotrigine in Han Chinese of Northern China. Eur J Clin Pharmacol 70:941–946
Dadheech S, Rao AV, Shaheen U et al (2013) Three most common nonsynonymous UGT1A6*2 polymorphisms (Thr181Ala, Arg184Ser and Ser7Ala) and therapeutic response to deferiprone in beta-thalassemia major patients. Gene 531:301–305
Guo D, Pang L, Han Y et al (2013) Polymorphisms of UGT1A9 and UGT2B7 influence the pharmacokinetics of mycophenolic acid after a single oral dose in healthy Chinese volunteers. Eur J Clin Pharmacol 69:843–849
He X, Hesse LM, Hazarika S et al (2009) Evidence for oxazepam as an in vivo probe of UGT2B15: oxazepam clearance is reduced by UGT2B15 D85Y polymorphism but unaffected by UGT2B17 deletion. Br J Clin Pharmacol 68:721–730
Ehmer U, Vogel A, Schutte JK et al (2004) Variation of hepatic glucuronidation: novel functional polymorphisms of the UDP-glucuronosyltransferase UGT1A4. Hepatology 39:970–977
Reimers A, Sjursen W, Helde G, Brodtkorb E (2014) Frequencies of UGT1A4*2 (P24T) and *3 (L48 V) and their effects on serum concentrations of lamotrigine. Eur J Drug Metab Pharmacokinet 41(2):149–155
Jain P, Shastri S, Gulati S et al (2015) Prevalence of UGT1A6 polymorphisms in children with epilepsy on valproate monotherapy. Neurol India 63:35–39
Ma L, Sun J, Peng Y et al (2012) Glucuronidation of edaravone by human liver and kidney microsomes: biphasic kinetics and identification of UGT1A9 as the major UDP-glucuronosyltransferase isoform. Drug Metab Dispos 40:734–741
Chung JY, Cho JY, Yu KS et al (2005) Effect of the UGT2B15 genotype on the pharmacokinetics, pharmacodynamics, and drug interactions of intravenous lorazepam in healthy volunteers. Clin Pharmacol Ther 77:486–494
Ma C, Wu X, Jiao Z et al (2015) SCN1A, ABCC2 and UGT2B7 gene polymorphisms in association with individualized oxcarbazepine therapy. Pharmacogenomics 16:347–360
Holthe M, Klepstad P, Zahlsen K, Borchgrevink PC, Hagen L et al (2002) Morphine glucuronide-to-morphine plasma ratios are unaffected by the UGT2B7 H268Y and UGT1A1*28 polymorphisms in cancer patients on chronic morphine therapy. Eur J Clin Pharmacol 58:353–356
Proposal for revised classification of epilepsies and epileptic syndromes (1989) Commission on classification and terminology of the international league against epilepsy. Epilepsia 30:389–399
Gonzalez-Esquivel DF, Ortega-Gavilan M, Alcantara-Lopez G, Jung-Cook H (2000) Plasma level monitoring of oxcarbazepine in epileptic patients. Arch Med Res 31:202–205
Park KJ, Kim JR, Joo EY et al (2012) Drug interaction and pharmacokinetic modeling of oxcarbazepine in korean patients with epilepsy. Clin Neuropharmacol 35:40–44
Sugiyama I, Murayama N, Kuroki A, Kota J, et al. (2015) Evaluation of cytochrome P450 inductions by anti-epileptic drug oxcarbazepine, 10-hydroxyoxcarbazepine, and carbamazepine using human hepatocytes and HepaRG cells. Xenobiotica 1–10
Zhang C, Zuo Z, Kwan P, Baum L (2011) In vitro transport profile of carbamazepine, oxcarbazepine, eslicarbazepine acetate, and their active metabolites by human P-glycoprotein. Epilepsia 52:1894–1904
Ma C-L, Wu X-Y, Zheng J, Wu HZ et al (2014) Association of SCN1A,SCN2A andABCC2 gene polymorphisms with the response to antiepileptic drugs in Chinese Han patients with epilepsy. Pharmacogenomics 15:1323–1336
Girard H, Villeneuve L, Court MH et al (2006) The novel UGT1A9 intronic I399 polymorphism appears as a predictor of 7-ethyl-10-hydroxycamptothecin glucuronidation levels in the liver. Drug Metab Dispos 34:1220–1228
Sandanaraj E, Jada SR, Shu X et al (2008) Influence of UGT1A9 intronic I399C > T polymorphism on SN-38 glucuronidation in Asian cancer patients. PHARMACOGENOMICS J 8:174–185
Saito Y, Sai K, Maekawa K et al (2009) Close association of UGT1A9 IVS1 + 399C > T with UGT1A1*28, *6, or *60 haplotype and its apparent influence on 7-ethyl-10-hydroxycamptothecin (SN-38) glucuronidation in Japanese. Drug Metab Dispos 37:272–276
Ho WF, Koo SH, Yee JY, Lee JD (2008) Genetic variations of the ABCC2 gene in the Chinese, Malay, and Indian populations of Singapore. Drug Metab Pharmacokinet 23:385–391
Innocenti F, Liu W, Chen P et al (2005) Haplotypes of variants in the UDP-glucuronosyltransferase1A9 and 1A1 genes. Pharmacogenet Genomics 15:295–301
Tukey RH, Strassburg CP (2000) Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol 40:581–616
Erickson-Ridout KK, Zhu J, Lazarus P (2011) Olanzapine metabolism and the significance of UGT1A448V and UGT2B1067Y variants. Pharmacogenet Genomics 21:539–551
Gulcebi MI, Ozkaynakci A, Goren MZ et al (2011) The relationship between UGT1A4 polymorphism and serum concentration of lamotrigine in patients with epilepsy. Epilepsy Res 95:1–8
Ciotti M, Marrone A, Potter C, Owens IS (1997) Genetic polymorphism in the human UGT1A6 (planar phenol) UDP-glucuronosyltransferase: pharmacological implications. Pharmacogenetics 7:485–495
Bock KW, Forster A, Gschaidmeier H et al (1993) Paracetamol glucuronidation by recombinant rat and human phenol UDP-glucuronosyltransferases. Biochem Pharmacol 45:1809–1814
Nagar S, Zalatoris JJ, Blanchard RL (2004) Human UGT1A6 pharmacogenetics: identification of a novel SNP, characterization of allele frequencies and functional analysis of recombinant allozymes in human liver tissue and in cultured cells. Pharmacogenetics 14:487–499
Limenta LM, Jirasomprasert T, Tankanitlert J et al (2008) UGT1A6 genotype-related pharmacokinetics of deferiprone (L1) in healthy volunteers. Br J Clin Pharmacol 65:908–916
Kuuranne T, Kurkela M, Thevis M et al (2003) Glucuronidation of anabolic androgenic steroids by recombinant human UDP-glucuronosyltransferases. Drug Metab Dispos 31:1117–1124
Zhang JY, Zhan J, Cook CS, Ings RM, Breau AP (2003) Involvement of human UGT2B7 and 2B15 in rofecoxib metabolism. Drug Metab Dispos 31:652–658
Stringer F, Scott G, Valbuena M et al (2013) The effect of genetic polymorphisms in UGT2B15 on the pharmacokinetic profile of sipoglitazar, a novel anti-diabetic agent. Eur J Clin Pharmacol 69:423–430
Acknowledgments
We sincerely thank the medical staff from Department of Neurology, Huashan Hospital, Fudan University, for their help and collaboration in the present study. This work was supported by the Natural Science Foundation of China [grant numbers 81671280].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest/disclosures
The authors declare that they have no financial or other conflicts of interest associated with this research and its publication.
Rights and permissions
About this article
Cite this article
Lu, Y., Fang, Y., Wu, X. et al. Effects of UGT1A9 genetic polymorphisms on monohydroxylated derivative of oxcarbazepine concentrations and oxcarbazepine monotherapeutic efficacy in Chinese patients with epilepsy. Eur J Clin Pharmacol 73, 307–315 (2017). https://doi.org/10.1007/s00228-016-2157-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-016-2157-3