Abstract
Purpose
Static and dynamic (PBPK) prediction models were applied to estimate the drug–drug interaction (DDI) risk of AZD2066. The predictions were compared to the results of an in vivo cocktail study. Various in vivo measures for tolbutamide as a probe agent for cytochrome P450 2C9 (CYP2C9) were also compared.
Methods
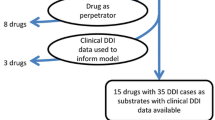
In vitro inhibition data for AZD2066 were obtained using human liver microsomes and CYP-specific probe substrates. DDI prediction was performed using PBPK modelling with the SimCYP simulator™ or static model. The cocktail study was an open label, baseline, controlled interaction study with 15 healthy volunteers receiving multiple doses of AD2066 for 12 days. A cocktail of single doses of 100 mg caffeine (CYP1A2 probe), 500 mg tolbutamide (CYP2C9 probe), 20 mg omeprazole (CYP2C19 probe) and 7.5 mg midazolam (CYP3A probe) was simultaneously applied at baseline and during the administration of AZD2066. Bupropion as a CYP2B6 probe (150 mg) and 100 mg metoprolol (CYP2D6 probe) were administered on separate days. The pharmacokinetic parameters for the probe drugs and their metabolites in plasma and urinary recovery were determined.
Results
In vitro AZD2066 inhibited CYP1A2, CYP2B6, CYP2C9, CYP2C19 and CYP2D6. The static model predicted in vivo interaction with predicted AUC ratio values of >1.1 for all CYP (except CYP3A4). The PBPK simulations predicted no risk for clinical relevant interactions. The cocktail study showed no interaction for the CYP2B6 and CYP2C19 enzymes, a possible weak inhibition of CYP1A2, CYP2C9 and CYP3A4 activities and a slight inhibition (29 %) of CYP2D6 activity. The tolbutamide phenotyping metrics indicated that there were significant correlations between CLform and AUCTOL, CL, Aemet and LnTOL24h. The MRAe in urine showed no correlation to CLform.
Conclusions
DDI prediction using the static approach based on total concentration indicated that AZD20066 has a potential risk for inhibition. However, no DDI risk could be predicted when a more in vivo-like dynamic prediction method with the PBPK with SimCYP™ software based on early human PK data was used and more parameters (i.e. free fraction in plasma, no DDI risk) were taken into account. The clinical cocktail study showed no or low risks for clinical relevant DDI interactions. Our findings are in line with the hypothesis that the dynamic prediction method predicts DDI in vivo in humans better than the static model based on total plasma concentrations.


Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the plasma time–concentration curve
- AUCR :
-
AUC ratio
- CL:
-
Clearance
- CLform :
-
Formation clearance
- fa :
-
Fraction absorbed
- fu :
-
Fraction unbound
- GLS:
-
Geometric least squares mean
- IC50 :
-
Inhibitor concentration at 50 % inhibition
- ka :
-
Absorption constant
- K i :
-
Inhibition constant
- lnTOL24h :
-
Concentration of tolbutamide at 24 h
- mGluR5:
-
Metabotropic glutamate receptor subtype 5
- MRAe :
-
Metabolic ratio in urine
- NPMH:
-
Neuropathic pain with mechanical hypersensitivity
- PBPK:
-
Physiologically based pharmacokinetic
- R:
-
AUC Ratio with and without inhibitor
- Vss :
-
Volume of distribution at steady state
References
Food and Drug Administration (2012) Draft guidance for industry—drug interaction studies, study design, data analysis, implications for dosing, and labeling recommendations. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM292362.pdf. Accessed: 28 Aug 2012
European Medicines Agency (2012) Guideline on the investigations of drug interactions. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/07/WC500129606.pdf. Accessed 23 Aug 2012
Bjornsson TD, Callaghan JT, Einolf HJ, Fischer V, Gan L, Grimm S, Kao J, King SP, Miwa G, Ni L, Kumar G, McLeod J, Obach SR, Roberts S, Roe A, Shah A, Snikeris F, Sullivan JT, Tweedie D, Vega JM, Walsh J, Wrighton SA, Pharmaceutical Research and Manufacturers of America Drug Metabolism/Clinical Pharmacology Technical Working Groups (2003) The conduct of in vitro and in vivo drug–drug interaction studies: a PhRMA perspective. J Clin Pharmacol 43:443–469
Guest EJ, Rowland-Yeo K, Rostami-Hodjegan A, Tucker GT, Houston JB, Galetin A (2011) Assessment of algorithms for predicting drug–drug interactions via inhibition mechanisms: comparison of dynamic and static models. Br J Clin Pharmacol 71:72–87
Rowland M, Peck C, Tucker G (2011) Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol 51:45–73
Zhao P, Zhang L, Grillo JA, Liu Q, Bullock JM, Moon YJ, Song P, Brar SS, Madabushi R, Wu TC, Booth BP, Rahman NA, Reynolds KS, Gil Berglund E, Lesko LJ, Huang SM (2011) Applications of physiologically based pharmacokinetic (PBPK) modeling and simulation during regulatory review. Clin Pharmacol Ther 89:259–267
Berg D, Godau J, Trenkwalder C, Eggert K, Csoti I, Storch A, Huber H, Morelli-Canelo M, Stamelou M, Ries V, Wolz M, Schneider C, Di Paolo T, Gasparini F, Hariry S, Vandemeulebroecke M, Abi-Saab W, Cooke K, Johns D, Gomez-Mancilla B (2011) AFQ056 treatment of levodopa-induced dyskinesias: results of 2 randomized controlled trials. Mov Disord 26:1243–1250
Levenga J, Hayashi S, de Vrij FM, Koekkoek SK, van der Linde HC, Nieuwenhuizen I, Song C, Buijsen RA, Pop AS, Gomezmancilla B, Nelson DL, Willemsen R, Gasparini F, Oostra BA (2011) AFQ056, a new mGluR5 antagonist for treatment of fragile X syndrome. Neurobiol Dis 42:311–317
Rohof WO, Lei A, Hirsch DP, Ny L, Astrand M, Hansen MB, Boeckxstaens GE (2012) The effects of a novel metabotropic glutamate receptor 5 antagonist (AZD2066) on transient lower oesophageal sphincter relaxations and reflux episodes in healthy volunteers. Aliment Pharmacol Ther 35:1231–1242
Jonzon B, Butler S, Karlsten R, Malamut R, Ståhle L, Huizar K, Stacey B (2012) Efficacy and safety of the mGluR5 antagonist AZD2066 in peripheral neuropathic pain patients with mechanical hypersensitivity (NPMH): results of a phase IIa randomised, double-blind, placebo-controlled study PT 427, 14th World Congress of Pain, IAPS
Karlsten R, Malamut R, Jonzon B, Ståhle L, Huizar K, Argoff C (2012) Efficacy and safety of the mGluR5 antagonist AZD2066 in painful diabetes neuropathy (PDN): results of a phase IIa randomised, double-blind, placebo-controlled study PT 429, 14th World Congress of Pain, IAPS
Ståhle L, Karlsten R, Jonzon B, Eriksson B, Kågedal M, Dominicus A (2012) Safety evaluation of the mGluR5 antagonists AZD9272, AZD2066 and AZD2516 in healthy volunteers and patients with neuropathic pain or major depressive disorder PT 444, 14th World Congress of Pain, IAPS
Jamei M, Marciniak S, Feng K, Barnett A, Tucker G, Rostami-Hodjegan A (2009) The Simcyp population-based ADME simulator. Expert Opin Drug Metab Toxicol 5:211–223
Blakey GE, Lockton JA, Perrett J, Norwood P, Russell M, Aherne Z, Plume J (2004) Pharmacokinetic and pharmacodynamic assessment of a five-probe metabolic cocktail for CYPs 1A2, 3A4, 2C9, 2D6 and 2E1. Br J Clin Pharmacol 57:162–169
Brandt RB, Laux JE, Yates SW (1987) Calculation of inhibitor Ki and inhibitor type from the concentration of inhibitor for 50% inhibition for Michaelis-Menten enzymes. Biochem Med Metab Biol 37:344–349
Ryu JY, Song IS, Sunwoo YE, Shon JH, Liu KH, Cha IJ, Shin JG (2007) Development of the "Inje cocktail" for high-throughput evaluation of five human cytochrome P450 isoforms in vivo. Clin Pharmacol Ther 82:531–540
Streetman DS, Bleakley JF, Kim JS, Nafziger AN, Leeder JS, Gaedigk A, Gotschall R, Kearns GL, Bertino JS Jr (2000) Combined phenotypic assessment of CYP1A2, CYP2C19, CYP2D6, CYP3A, N-acetyltransferase-2, and xanthine oxidase with the "Cooperstown cocktail". Clin Pharmacol Ther 68:375–383
Chainuvati S, Nafziger AN, Leeder JS, Gaedigk A, Kearns GL, Sellers E, Zhang Y, Kashuba AD, Rowland E, Bertino JS Jr (2003) Combined phenotypic assessment of cytochrome p450 1A2, 2C9, 2C19, 2D6, and 3A, N-acetyltransferase-2, and xanthine oxidase activities with the "Cooperstown 5+1 cocktail". Clin Pharmacol Ther 74:437–447
Christensen M, Andersson K, Dalen P, Mirghani RA, Muirhead GJ, Nordmark A, Tybring G, Wahlberg A, Yasar U, Bertilsson L (2003) The Karolinska cocktail for phenotyping of five human cytochrome P450 enzymes. Clin Pharmacol Ther 73:517–528
Rostami-Hodjegan A, Nurminen S, Jackson PR, Tucker GT (1996) Caffeine urinary metabolite ratios as markers of enzyme activity: a theoretical assessment. Pharmacogenetics 6:121–149
Fuhr U, Rost KL (1994) Simple and reliable CYP1A2 phenotyping by the paraxanthine/caffeine ratio in plasma and in saliva. Pharmacogenetics 4:109–116
Fuhr U, Rost KL, Engelhardt R, Sachs M, Liermann D, Belloc C, Beaune P, Janezic S, Grant D, Meyer UA, Staib AH (1996) Evaluation of caffeine as a test drug for CYP1A2, NAT2 and CYP2E1 phenotyping in man by in vivo versus in vitro correlations. Pharmacogenetics 6:159–176
Faucette SR, Hawke RL, Lecluyse EL, Shord SS, Yan B, Laethem RM, Lindley CM (2000) Validation of bupropion hydroxylation as a selective marker of human cytochrome P450 2B6 catalytic activity. Drug Metab Dispos 28:1222–1230
Loboz KK, Gross AS, Williams KM, Liauw WS, Day RO, Blievernicht JK, Zanger UM, McLachlan AJ (2006) Cytochrome P450 2B6 activity as measured by bupropion hydroxylation: effect of induction by rifampin and ethnicity. Clin Pharmacol Ther 80:75–84
Palovaara S, Pelkonen O, Uusitalo J, Lundgren S, Laine K (2003) Inhibition of cytochrome P450 2B6 activity by hormone replacement therapy and oral contraceptive as measured by bupropion hydroxylation. Clin Pharmacol Ther 74:326–333
Turpeinen M, Tolonen A, Uusitalo J, Jalonen J, Pelkonen O, Laine K (2005) Effect of clopidogrel and ticlopidine on cytochrome P450 2B6 activity as measured by bupropion hydroxylation. Clin Pharmacol Ther 77:553–559
Kirchheiner J, Roots I, Goldammer M, Rosenkranz B, Brockmoller J (2005) Effect of genetic polymorphisms in cytochrome p450 (CYP) 2C9 and CYP2C8 on the pharmacokinetics of oral antidiabetic drugs: clinical relevance. Clin Pharmacokinet 44:1209–1225
Shon JH, Yoon YR, Kim KA, Lim YC, Lee KJ, Park JY, Cha IJ, Flockhart DA, Shin JG (2002) Effects of CYP2C19 and CYP2C9 genetic polymorphisms on the disposition of and blood glucose lowering response to tolbutamide in humans. Pharmacogenetics 12:111–119
Lee CR, Pieper JA, Frye RF, Hinderliter AL, Blaisdell JA, Goldstein JA (2003) Tolbutamide, flurbiprofen, and losartan as probes of CYP2C9 activity in humans. J Clin Pharmacol 43:84–91
Lee CR, Hawke RL, Pieper JA (2005) Twenty-four hour tolbutamide plasma concentration as a phenotypic measure of CYP2C9 activity. Eur J Clin Pharmacol 61:315–316
Veronese ME, Miners JO, Randles D, Gregov D, Birkett DJ (1990) Validation of the tolbutamide metabolic ratio for population screening with use of sulfaphenazole to produce model phenotypic poor metabolizers. Clin Pharmacol Ther 47:403–411
Jetter A, Kinzig-Schippers M, Skott A, Lazar A, Tomalik-Scharte D, Kirchheiner J, Walchner-Bonjean M, Hering U, Jakob V, Rodamer M, Jabrane W, Kasel D, Brockmoller J, Fuhr U, Sorgel F (2004) Cytochrome P450 2C9 phenotyping using low-dose tolbutamide. Eur J Clin Pharmacol 60:165–171
Lee CR, Pieper JA, Hinderliter AL, Blaisdell JA, Goldstein JA (2002) Evaluation of cytochrome P4502C9 metabolic activity with tolbutamide in CYP2C91 heterozygotes. Clin Pharmacol Ther 72:562–571
Chang M, Dahl ML, Tybring G, Gotharson E, Bertilsson L (1995) Use of omeprazole as a probe drug for CYP2C19 phenotype in Swedish Caucasians: comparison with S-mephenytoin hydroxylation phenotype and CYP2C19 genotype. Pharmacogenetics 5:358–363
Niioka T, Uno T, Sugimoto K, Sugawara K, Hayakari M, Tateishi T (2007) Estimation of CYP2C19 activity by the omeprazole hydroxylation index at a single point in time after intravenous and oral administration. Eur J Clin Pharmacol 63:1031–1038
Tamminga WJ, Wemer J, Oosterhuis B, Brakenhoff JP, Gerrits MG, de Zeeuw RA, de Leij LF, Jonkman JH (2001) An optimized methodology for combined phenotyping and genotyping on CYP2D6 and CYP2C19. Eur J Clin Pharmacol 57:143–146
Wandel C, Bocker RH, Bohrer H, deVries JX, Hofmann W, Walter K, Kleingeist B, Neff S, Ding R, Walter-Sack I, Martin E (1998) Relationship between hepatic cytochrome P450 3A content and activity and the disposition of midazolam administered orally. Drug Metab Dispos 26:110–114
Gorski JC, Jones DR, Haehner-Daniels BD, Hamman MA, O'Mara EM Jr, Hall SD (1998) The contribution of intestinal and hepatic CYP3A to the interaction between midazolam and clarithromycin. Clin Pharmacol Ther 64:133–143
Gorski JC, Hall SD, Jones DR, VandenBranden M, Wrighton SA (1994) Regioselective biotransformation of midazolam by members of the human cytochrome P450 3A (CYP3A) subfamily. Biochem Pharmacol 47:1643–1653
Thummel KE, Shen DD, Podoll TD, Kunze KL, Trager WF, Hartwell PS, Raisys VA, Marsh CL, McVicar JP, Barr DM (1994) Use of midazolam as a human cytochrome P450 3A probe: I. In vitro-in vivo correlations in liver transplant patients. J Pharmacol Exp Ther 271:549–556
Zhao P, Ragueneau-Majlessi I, Zhang L, Strong JM, Reynolds KS, Levy RH, Thummel KE, Huang SM (2009) Quantitative evaluation of pharmacokinetic inhibition of CYP3A substrates by ketoconazole: a simulation study. J Clin Pharmacol 49:351–359
Peters SA, Schroeder P, Giri N, Dolgos H (2012) Evaluation of the use of static and dynamic models to predict drug-drug interaction and its associated variability: impact on drug discovery and early development. Drug Metab Dispos 40(8):1495–1507
Yang Z, Vakkalagadda B, Shen G, Ahlers CM, Has T, Christopher LJ, Kurland JF, Roongta V, Masson E, Zhang S (2012) Inhibitory effect of ketoconazole on the pharmacokinetics of amMultireceptor tyrosine Kinase inhibitor BMS-690514 in healthy participants: assessing the mechanism of the interaction with physiologically-based pharmacokinetic simulations. J Clin Pharmacol. doi:10.1177/0091270011439208
Acknowledgements
The authors are grateful to Jenny Aasa and Elin Sohlberg for their assistance in the in vitro experiments and part of the SimCYP simulations. We also would like to thank Urban Fagerholm and all other AstraZeneca scientists involved for their assistance. The personnel at ICON Development Solution and PRA International are thanked for their involvement in this study.
Conflict of interest
This research was funded by AstraZeneca. All authors are or were employees at AstraZeneca.
Author information
Authors and Affiliations
Corresponding author
Additional information
The views expressed in this article are those of the authors and do not reflect official views of the Medical Products Agency
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 212 kb)
Rights and permissions
About this article
Cite this article
Nordmark, A., Andersson, A., Baranczewski, P. et al. Assessment of interaction potential of AZD2066 using in vitro metabolism tools, physiologically based pharmacokinetic modelling and in vivo cocktail data. Eur J Clin Pharmacol 70, 167–178 (2014). https://doi.org/10.1007/s00228-013-1603-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-013-1603-8