Abstract
Objectives
To estimate the pharmacokinetic (PK) properties of posaconazole in patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) undergoing chemotherapy in a clinical setting.
Methods
Posaconazole concentrations in patients with AML/MDS receiving prophylactic posaconazole were determined by high-performance liquid chromatography. A population PK model with nonlinear mixed effect modeling was developed. The list of tested covariates included age, weight, height, gender, posaconazole dose, ethnicity, co-administration of antineoplastic chemotherapy, ranitidine or pantoprazole, coincident fever, diarrhea, leukocyte counts, and γ-glutamyltransterase plasma activity.
Results
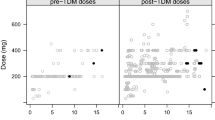
A total of 643 serum concentrations of posaconazole from 84 patients were obtained. A one-compartment model with first order absorption and elimination as the basic structural model appropriately described the data, with an apparent clearance of 56.8 L/h [95% confidence interval (CI) 52.8–60.8 L/h] and an apparent volume of distribution of 2,130 L (95% CI 1,646–2,614 L). Significant effects on apparent clearance (CL/F) were found for presence of diarrhea and for co-medication with proton-pump inhibitors (1.5- and 1.6-fold increase in CL/F, respectively), weight (33.4 L larger apparent volume of distribution per kilogram), and co-administration of chemotherapy (0.6-fold lower apparent volume of distribution).
Conclusion
We developed a prediction basis for mean posaconazole concentrations in AML/MDS patients. Patient weight, presence of diarrhea, and concomitant medication (chemotherapy and pantoprazole) showed significant effects on posaconazole exposure. Corresponding adjustments of the starting dose according to the presence of diarrhea and during the co-administration of chemotherapy or proton-pump inhibitors appear justified before therapeutic drug monitoring results are available. Further investigation of the interaction between different chemotherapeutic regimens and posaconazole is warranted.

Similar content being viewed by others
References
Lass-Florl C (2009) The changing face of epidemiology of invasive fungal disease in Europe. Mycoses 52(3):197–205
Vehreschild JJ, Rüping MJGT, Wisplinghoff H, Farowski F, Steinbach A, Sims R, Stollorz A, Kreuzer K-A, Hallek M, Bangard C, Cornely OA (2010) Clinical effectiveness of posaconazole prophylaxis in patients with acute myelogenous leukemia (AML): A six year experience of the Cologne AML Cohort. J Antimicrob Chemother 65:1466–1471
Cuenca-Estrella M, Bernal-Martinez L, Buitrago MJ, Castelli MV, Gomez-Lopez A, Zaragoza O, Rodriguez-Tudela JL (2008) Update on the epidemiology and diagnosis of invasive fungal infection. Int J Antimicrob Agents 32[Suppl 2]:S143–S147. doi:10.1016/S0924-8579(08)70016-5
Reboli AC, Rotstein C, Pappas PG, Chapman SW, Kett DH, Kumar D, Betts R, Wible M, Goldstein BP, Schranz J, Krause DS, Walsh TJ (2007) Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med 356(24):2472–2482. doi:10.1056/NEJMoa066906
Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, Kern WV, Marr KA, Ribaud P, Lortholary O, Sylvester R, Rubin RH, Wingard JR, Stark P, Durand C, Caillot D, Thiel E, Chandrasekar PH, Hodges MR, Schlamm HT, Troke PF, de Pauw B (2002) Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 347(6):408–415
Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, Greinix H, Morais de Azevedo W, Reddy V, Boparai N, Pedicone L, Patino H, Durrant S (2007) Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med 356(4):335–347
Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, Helfgott D, Holowiecki J, Stockelberg D, Goh YT, Petrini M, Hardalo C, Suresh R, Angulo-Gonzalez D (2007) Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 356(4):348–359. doi:10.1056/NEJMoa061094
Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik JA, Wingard JR, Patterson TF (2008) Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 46(3):327–360. doi:10.1086/525258
European LeukemiaNet, European Group for Blood & Marrow Transplantation, European Organization for Research and Treatment of Cancer and International Immunocompromised Host Society (2007). 2007 Update of the ECIL-1 guidelines for antifungal prophylaxis in leukemia patients, including allogeneic HSCT recipients. Available at: http://www.ichs.org/Ecilslides/ECIL2%20%20Antifungal%20prophylaxis%20update%202007.pdf. Accessed 9 Mar 2010
Cornely OA, Bohme A, Buchheidt D, Einsele H, Heinz WJ, Karthaus M, Krause SW, Kruger W, Maschmeyer G, Penack O, Ritter J, Ruhnke M, Sandherr M, Sieniawski M, Vehreschild JJ, Wolf HH, Ullmann AJ (2009) Primary prophylaxis of invasive fungal infections in patients with hematologic malignancies. Recommendations of the infectious diseases working party of the German society for haematology and oncology. Haematologica 94(1):113–122. doi:10.3324/haematol.11665
National Comprehensive Cancer Network (2009) National Comprehensive Cancer Network clinical practice guidelines in oncology: prevention and treatment of cancer-related Infections v.2.2009. Available at: http://www.nccn.org/professionals/physician_gls/PDF/infections.pdf. Accessed 8 Apr 2010
Lipp HP (2011) Posaconazole: clinical pharmacokinetics and drug interactions. Mycoses 54:32–38. doi:10.1111/j.1439-0507.2010.01984.x
Farowski F, Vehreschild J, Cornely OA (2007) Posaconazole: a next-generation triazole antifungal. Future Microbiol 2(3):231–243. doi:10.2217/17460913.2.3.231
Rodriguez MM, Pastor FJ, Calvo E, Salas V, Sutton DA, Guarro J (2009) Correlation of in vitro activity, serum levels, and in vivo efficacy of posaconazole against Rhizopus microsporus in a murine disseminated infection. Antimicrob Agents Chemother 53(12):5022–5025. doi:10.1128/AAC.01026-09
Kishel JJ, Sivik J (2008) Breakthrough invasive fungal infection in an immunocompromised host while on posaconazole prophylaxis: an omission in patient counseling and follow-up. J Oncol Pharm Pract 14(4):189–193. doi:10.1177/1078155208094123
Krishna G, Martinho M, Chandrasekar P, Ullmann AJ, Patino H (2007) Pharmacokinetics of oral posaconazole in allogeneic hematopoietic stem cell transplant recipients with graft-versus-host disease. Pharmacotherapy 27(12):1627–1636. doi:10.1592/phco.27.12.1627
Krishna G, AbuTarif M, Xuan F, Martinho M, Angulo D, Cornely OA (2008) Pharmacokinetics of oral posaconazole in neutropenic patients receiving chemotherapy for acute myelogenous leukemia or myelodysplastic syndrome. Pharmacotherapy 28(10):1223–1232
Jang SH, Colangelo PM, Gobburu JV (2010) Exposure-response of posaconazole used for prophylaxis against invasive fungal infections: evaluating the need to adjust doses based on drug concentrations in plasma. Clin Pharmacol Ther 88(1):115–119. doi:10.1038/clpt.2010.64
Krishna G, Ma L, Vickery D, Yu X, Wu I, Power E, Beresford E, Komjathy S (2009) Effect of varying amounts of a liquid nutritional supplement on the pharmacokinetics of posaconazole in healthy volunteers. Antimicrob Agents Chemother 53(11):4749–4752. doi:10.1128/AAC.00889-09
Kohl V, Muller C, Cornely OA, Abduljalil K, Fuhr U, Vehreschild JJ, Scheid C, Hallek M, Ruping MJ (2010) Factors influencing pharmacokinetics of prophylactic posaconazole in patients undergoing allogeneic stem cell transplantation. Antimicrob Agents Chemother 54(1):207–212. doi:10.1128/AAC.01027-09
AbuTarif MA, Krishna G, Statkevich P (2010) Population pharmacokinetics of posaconazole in neutropenic patients receiving chemotherapy for acute myelogenous leukemia or myelodysplastic syndrome. Curr Med Res Opin 26(2):397–405. doi:10.1185/03007990903485056
Muller C, Arndt M, Queckenberg C, Cornely OA, Theisohn M (2006) HPLC analysis of the antifungal agent posaconazole in patients with haematological diseases. Mycoses 49[Suppl 1]:17–22. doi:10.1111/j.1439-0507.2006.01297.x
Lindbom L, Pihlgren P, Jonsson EN (2005) PsN-Toolkit–a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed 79(3):241–257. doi:10.1016/j.cmpb.2005.04.005
Lebeaux D, Lanternier F, Elie C, Suarez F, Buzyn A, Viard JP, Bougnoux ME, Lecuit M, Jullien V, Lortholary O (2009) Therapeutic drug monitoring of posaconazole: a monocentric study with 54 adults. Antimicrob Agents Chemother 53(12):5224–5229. doi:10.1128/AAC.00939-09
Thompson GR 3rd, Rinaldi MG, Pennick G, Dorsey SA, Patterson TF, Lewis JS 2nd (2009) Posaconazole therapeutic drug monitoring: a reference laboratory experience. Antimicrob Agents Chemother 53(5):2223–2224. doi:10.1128/AAC.00240-09
Neubauer WC, Engelhardt M, Konig A, Hieke S, Jung M, Bertz H, Kummerer K (2010) Therapeutic drug monitoring of posaconazole in hematology patients: experience with a new high-performance liquid chromatography-based method. Antimicrob Agents Chemother 54(9):4029–4032. doi:10.1128/AAC.00150-10
Cornely OA, Helfgott D, Krishna G, Carmelitano P, Martinho M, McCarthy M (2010) Pharmacokinetics of different dosing strategies of oral posaconazole, poster A1-042. In: Interscience Conf Antimicrobial Agents and Chemotherapy. Boston
Krishna G, Moton A, Ma L, Medlock MM, McLeod J (2009) Pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob Agents Ch 53(3):958–966. doi:10.1128/Aac.01034-08
AbuTarif MA, Krishna G, Statkevich P (2010) Population pharmacokinetics of posaconazole in neutropenic patients receiving chemotherapy for acute myelogenous leukemia or myelodysplastic syndrome. Curr Med Res Opin 26(2):397–405. doi:10.1185/03007990903485056
Shields RK, Clancy CJ, Vadnerkar A, Kwak EJ, Silveira FP, Massih RCA, Pilewski JM, Crespo M, Toyoda Y, Bhama JK, Bermudez C, Nguyen MH (2011) Posaconazole serum concentrations among cardiothoracic transplant recipients: factors impacting trough levels and correlation with clinical response to therapy. Antimicrob Agents Ch 55(3):1308–1311. doi:10.1128/Aac.01325-10
Alffenaar JWC, van Assen S, van der Werf TS, Kosterink JGW, Uges DRA (2009) Omeprazole significantly reduces posaconazole serum trough level. Clin Infect Dis 48(6):839–839. doi:10.1086/597110
Farowski F, Cornely OA, Vehreschild JJ, Hartmann P, Bauer T, Steinbach A, Rüping MJGT, Müller C (2010) Intracellular concentrations of posaconazole in different compartments of the peripheral blood. Antimicrob Agents Chemother 54:2928–2931
Cornely OA, Helfgott D, Krishna G, Ma L, Carmelitano P, Martinho M, McCarthy M (2010) Pharmacokinetics of different dosing strategies of oral posaconazole, poster 1237). ICAAC, Boston
Transparency declaration
Carsten Müller, Farowski Fedja, Uwe Fuhr, Michael Hallek, and Victoria Kohl have no conflicts to declare.
JJV has served on the speakers’ bureau of Astellas, Gilead, Merck/Schering-Plough, and Pfizer, and received research grants from Merck/Schering-Plough and Pfizer.
Maria Vehreschild has served on the speakers’ bureau of Astellas, Gilead, Merck/Schering-Plough and Pfizer.
Oliver A Cornely is supported by the German Federal Ministry of Research and Education (BMBF grant 01KN0706), has received research grants from Actelion, Astellas, Basilea, Bayer, Biocryst, Celgene, F2G, Genzyme, Gilead, Merck/Schering, Miltenyi, Optimer, Pfizer, Quintiles, and Viropharma, is a consultant to Astellas, Basilea, F2G, Gilead, Merck/Schering, Mölnlycke, Optimer, and Pfizer, and has received lecture honoraria from Astellas, Gilead, Merck/Schering, and Pfizer.
KAK is a consultant to and has received financial or material support from Merck.
Funding
No funding of any kind was received for this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vehreschild, J.J., Müller, C., Farowski, F. et al. Factors influencing the pharmacokinetics of prophylactic posaconazole oral suspension in patients with acute myeloid leukemia or myelodysplastic syndrome. Eur J Clin Pharmacol 68, 987–995 (2012). https://doi.org/10.1007/s00228-012-1212-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1212-y