Abstract
Aim
To describe the pattern of drug use among Chinese women during the first trimester and to examine the impact of maternal diseases on the choice of drugs.
Method
This drug utilisation study of pregnant women was performed using data from the ABCD cohort study. A total of 4,290 women were enrolled in the analysis. Information was collected by self-completion questionnaire combined with the “Maternal health handbook”.
Results
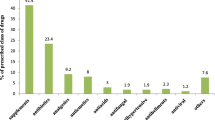
Of the 4,290 women interviewed, 75.9% of women took at least one drug during the first trimester. Users took a mean number of 1.43 drugs. The most frequently used drugs were folic acid (65.2%), vitamins (14.6%), calcium (12.0%), minerals (11.1%), Chinese traditional patent medicine (CTPM; 10.1%) and anti-infectives (6.5%). Among the women having used CTPM, influenza/cold and threatened abortion were the most commonly reported indications. Logistic regression analysis of drug use (excluding nutritional and haematological drugs) shows that CTPM and Western medicine are both associated with the use of drugs for occasional diseases and against threatened abortion. Maternal chronic diseases were not associated with the use of CTPM.
Conclusion
This analysis of pregnant women showed that drugs were prescribed to most women, even when nutritional and haematological drugs were excluded. Our data reflect, except for drugs used for chronic diseases, a general reluctance among Chinese women to use Western medicine and resorting to CTPM during pregnancy
Similar content being viewed by others
References
Källén BA (2005) Methodological issues in the epidemiological study of the teratogenicity of drugs. Congenit Anom (Kyoto) 45:44–51
Gagne JJ, Maio V, Berghella V, Louis DZ, Gonnella JS (2008) Prescription drug use during pregnancy: a population-based study in Regione Emilia-Romagna, Italy. Eur J Clin Pharmacol 64:1125–1132
Headley J, Northstone K, Simmons H, Golding J, ALSPAC Study Team (2004) Medication use during pregnancy: data from the Avon Longitudinal Study of Parents and Children. Eur J Clin Pharmacol 60:355–361
Egen-Lappe V, Hasford J (2004) Drug prescription in pregnancy: analysis of a large statutory sickness fund population. Eur J Clin Pharmacol 60:659–666
Sharma R, Kapoor B, Verma U (2006) Drug utilization pattern during pregnancy in North India. Indian J Med Sci 60:277–287
De Vigan C, De Walle HE, Cordier S, Goujard J, Knill-Jones R, Aymé S, Calzolari E, Bianchi F (1999) Therapeutic drug use during pregnancy: a comparison in four European countries. OECM Working Group. Occupational Exposures and Congenital Anomalies. J Clin Epidemiol 52:977–982
Lacroix I, Damase-Michel C, Lapeyre-Mestre M, Montastruc JL (2000) Prescription of drugs during pregnancy in France. Lancet 356:1735–1736
Bakker MK, Jentink J, Vroom F, Van Den Berg PB, De Walle HE, De Jong-Van Den Berg LT (2006) Drug prescription pattern before, during and after pregnancy for chronic, occasional and pregnancy-related drugs in the Netherlands. BJOG 113:559–568
Lacroix I, Hurault C, Sarramon MF, Guitard C, Berrebi A, Grau M, Albouy-Cossard C, Bourrel R, Elefant E, Montastruc JL, Damase-Michel C (2009) Prescription of drugs during pregnancy: a study using EFEMERIS, the new French database. Eur J Clin Pharmacol 65:839–846
Donati S, Baglio G, Spinelli A, Grandolfo ME (2000) Drug use in pregnancy among Italian women. Eur J Clin Pharmacol 56:323–328
Engeland A, Bramness JG, Daltveit AK, Rønning M, Skurtveit S, Furu K (2008) Prescription drug use among fathers and mothers before and during pregnancy. A population-based cohort study of 106,000 pregnancies in Norway 2004–2006. Br J Clin Pharmacol 65:653–660
Hardy JB (2003) The Collaborative Perinatal Project: lessons and legacy. Ann Epidemiol 13:303–311
Blumenthal D, Hsiao W (2005) Privatization and its discontents—the evolving Chinese health care system. N Engl J Med 353:1165–1170
Checa MA, Peiró R, Pascual J, Carreras R (2005) Drug intake behaviour of immigrants during pregnancy. Eur J Obstet Gynecol Reprod Biol 121:38–45
[No authors listed] (1996) Complementary medicine is booming worldwide. BMJ 313:131–133
Ko RJ (1998) Adulterants in Asian patent medicines. N Engl J Med 339:847
Moretti M (2007) Collection and analysis of drug safety data in pregnancy. Can J Clin Pharmacol 14:e34–e36
Paganini-Hill A, Ross RK (1982) Reliability of recall of drug usage and other health-related information. Am J Epidemiol 116:114–122
Mitchell AA, Cottler LB, Shapiro S (1986) Effect of questionnaire design on recall of drug exposure in pregnancy. Am J Epidemiol 123:670–676
Li S, Moore CA, Li Z, Berry RJ, Gindler J, Hong SX, Liu Y, Mulinare J, Wong LY, Gu HQ, Erickson JD (2003) A population-based birth defects surveillance system in the People’s Republic of China. Paediatr Perinat Epidemiol 17:287–293
Chuang CH, Hsieh WS, Guo YL, Tsai YJ, Chang PJ, Lin SJ, Chen PC (2007) Chinese herbal medicines used in pregnancy: a population-based survey in Taiwan. Pharmacoepidemiol Drug Saf 16:464–468
Leung KY, Lee YP, Chan HY, Lee CP, Tang MH (2002) Are herbal medicinal products less teratogenic than Western pharmaceutical products. Acta Pharmacol Sin 23:1169–1172
Chuang CH, Chang PJ, Hsieh WS, Tsai YJ, Lin SJ, Chen PC (2009) Chinese herbal medicine use in Taiwan during pregnancy and the postpartum period: a population-based cohort study. Int J Nurs Stud 46:787–795
Acknowledgements
This research was supported financially by the China National Key Technology Research and Development Program 2006BAI05A03. We are extremely grateful to all the women who took part and to all the health doctors for their co-operation and help in recruitment. All authors contributed to the writing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, X., Qi, X., Hao, J. et al. Pattern of drug use during the first trimester among Chinese women: data from a population-based cohort study. Eur J Clin Pharmacol 66, 511–518 (2010). https://doi.org/10.1007/s00228-009-0781-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-009-0781-x