Abstract
Objective
The aim of the present study was to evaluate a computerised monitoring system (CMS) based on laboratory test results for the detection of adverse drug reactions (ADRs) on a paediatric ward.
Methods
A prospective, 6-month pharmacoepidemiological survey was performed on a 22-bed paediatric isolation ward. ADRs were identified by intensive chart review. In addition to spontaneous reporting by the treating physician, automatic laboratory signals generated by a CMS were evaluated for their association with ADRs. ADRs were classified by the affected target organs according to the WHO–ART system organ classes.
Results
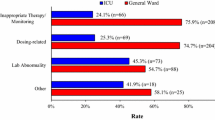
A total of 73 ADRs were identified in 439 admissions (396 patients) by chart review. The CMS alerted 31 (42.4%) ADRs while 23 (31.5%) ADRs were found solely by treating physicians. Eight ADRs were detected by both approaches resulting in a total detection rate of 74% (compared with intensive pharmacovigilance). Out of a total of 27,434 laboratory tests performed routinely, 1,563 were classified as abnormal by the predefined CMS and used as the basis of alerts. The sensitivity of the system with respect to patients alerted was 90.3% and the specificity only 19.6%.
Conclusion
This study demonstrates that, using CMS, a different kind of mild adverse events were detected compared to the observation by the treating physician. The system presented appears to be sufficiently sensitive, but the specificity is too low to make it acceptable for physicians in daily practice. In children, clinically important ADRs can be detected best by intensified surveillance.
Similar content being viewed by others
References
Azaz-Livshits T, Levy M, Sadan B, Shalit M, Geisslinger G, Brune K (1998) Computerized survelliance of adverse drug reactions in hospital: pilot study. Br J Clin Pharmacol 45:309–314
Bates DW, Evans RS, Murff H, Stetson PD, Pizziferri L, Hripcsak G (2003) Detecting adverse events using information technology. J Am Med Inform Assoc 10:115–128
Classen DC, Pestotnik SL, Evans RS, Burke JP (1991) Computerized surveillance of adverse drug events in hospital patients. JAMA 266:2847–2851
Dormann H, Criegee-Rieck M, Neubert A, Egger T, Levy M, Hahn EG et al (2004) Implementation of a computer-assisted monitoring system for the detection of adverse drug reactions in gastroenterology. Aliment Pharmacol Ther 19:303–309
Dormann H, Muth-Selbach U, Krebs S, Criegee-Rieck M, Tegeder I, Schneider HT et al (2000) Incidence and costs of adverse drug reactions during hospitalisation: computerised monitoring versus stimulated spontaneous reporting. Drug Saf 22:161–168
Forsstrom JJ, Gronroos P, Irjala K, Heiskanen J, Torniainen K (1996) Linking patient medication data with laboratory information system. Int J Biomed Comput 42:111–116
Haffner S, von Laue N, Wirth S, Thurmann PA (2005) Detecting adverse drug reactions on paediatric wards: intensified surveillance versus computerised screening of laboratory values. Drug Saf 28:453–464
Honigman B, Light P, Pulling RM, Bates DW (2001) A computerized method for identifying incidents associated with adverse drug events in outpatients. Int J Med Inf 61:21–32
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA et al (1981) A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 30:239–245
Ruggeri A, CFMA (1996) Development and evaluation of a knowledge-based system to assess a drug’s safety profile from laboratory data. Drug Inf J 30:413–419
Schiff GD, Klass D, Peterson J, Shah G, Bates DW (2003) Linking laboratory and pharmacy: opportunities for reducing errors and improving care. Arch Intern Med 163:893–900
Weiss J, Krebs S, Hoffmann C, Werner U, Neubert A, Brune K et al (2002) Survey of adverse drug reactions on a pediatric ward: a strategy for early and detailed detection. Pediatrics 110:254–257
Acknowledgments
This study was supported by grants of German Israeli Foundation, Bayerisches Staatsministerium and Marohn Stiftung.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Neubert, A., Dormann, H., Weiss, J. et al. Are computerised monitoring systems of value to improve pharmacovigilance in paediatric patients?. Eur J Clin Pharmacol 62, 959–965 (2006). https://doi.org/10.1007/s00228-006-0197-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-006-0197-9