Abstract
Objectives
To determine whether caffeine (CA) clearance and salivary paraxanthine (PX)/CA ratio were altered in alcohol-dependent subjects and also whether salivary PX/CA ratio was affected by arylamine-N-acetylation 2 (NAT-2) genotypes.
Methods
Of 30 male individuals recruited in assessing PX/CA ratio and CA clearance by ingestion of CA, 13 were healthy control, 10 were alcohol-dependent subjects with abnormal liver function tests and 7 were alcohol-dependent subjects with normal liver function. CA and PX levels were analysed by means of high-performance liquid chromatography. CA clearance was calculated from concentrations of CA in saliva at various time intervals. In another study, PX/CA ratios were assessed in 46 healthy male subjects. Their NAT2 status was genotyped using polymerase chain reaction with restriction fragment length polymorphism assay.
Results
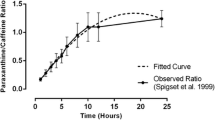
Salivary CA clearance was well correlated with plasma and salivary PX/CA ratios in a wide range of the clearance. Correlation between salivary PX/CA ratio and CA clearance was considered high from the first hour after CA ingestion and continued so for at least 9 h (r=0.94–0.96, P<0.001). PX/CA ratio in saliva was also well correlated with plasma PX/CA ratio (r=0.98, P<0.001). Salivary CA clearance in the control group was significantly higher than that of patients with abnormal liver function tests, i.e. (mean±SEM; 95% confidence limits; l/h/kg) 0.094±0.013 (0.064–0.124) and 0.044±0.019 (0.002–0.091), respectively, and not different from that of patients with normal liver function tests [0.107±0.017 (0.066–0.149)]. Similarly, the same is true for PX/CA ratio. NAT2 genotype status did not apparently affect PX/CA ratio.
Conclusion
Saliva-based PX/CA ratio was a convenient and robust method for assessment of CYP1A2 activity and liver function and it was shown to be altered in alcohol-dependent patients with mild abnormal liver function test.



Similar content being viewed by others
References
Masellis M, Basile VS, Ozdemir V, Meltzer HY, Macciardi FM, Kennedy JL (2000) Pharmacogenetics of antipsychotic treatment: lessons learned from clozapine. Biol Psychiatry 47:252–266
Basile VS, Ozdemir V, Masellis M, Walker ML, Meltzer HY, Lieberman JA, Potkin SG, Alva G, Kalow W, Macciardi FM, Kennedy JL (2000) A functional polymorphism of the cytochrome P450 1A2 (CYP1A2) gene: association with tardive dyskinesia in schizophrenia. Mol Psychiatry 5:410–417
Landi MT, Sinha R, Lang NP, Kadlubar FF (1999) Human cytochrome P4501A2. In: Ryder W (ed) Metabolic polymorphism to cancer. IARC Sci Publ no. 148. International Agency for Research in Cancer, Lyon, pp 173–195
Campbell ME, Spielberg SP, Kalow W (1987) A urinary metabolite ratio that reflects systemic caffeine clearance. Clin Pharmacol Ther 42:157–165
Rasmussen BB, Brix TH, Kyvik KO, Brosen K (2002) The interindividual differences in the 3-demthylation of caffeine alias CYP1A2 is determined by both genetic and environmental factors. Pharmacogenetics 12:473–478
Tang BK, Zhou Y, Kadar D, Kalow W (1994) Caffeine as a probe for CYP1A2 activity: potential influence of renal factors on urinary phenotypic trait measurements. Pharmacogenetics 4:117–124
Rost KL, Brosicke H, Heinemeyer G, Roots I (1994) Specific and dose-dependent enzyme induction by omeprazole in human beings. Hepatology 20:1204–1212
Brosen K, Skjelbo E, Rasmussen BB, Poulsen HE, Loft S (1993) Fluvoxamine is a potent inhibitor of cytochrome P4501A2. Biochem Pharmacol 45:1211–1214
Eaton DL, Gallagher EP, Bammler TK, Kunze KL (1995) Role of cytochrome P4501A2 in chemical carcinogenesis: implications for human variability in expression and enzyme activity. Pharmacogenetics 5:259–274
Lang NP, Butler MA, Massengill J, Lawson M, Stotts RC, Hauer Jensen M, Kadlubar FF (1994) Rapid metabolic phenotypes for acetyltransferase and cytochrome P4501A2 and putative exposure to food-borne heterocyclic amines increase the risk for colorectal cancer or polyps. Cancer Epidemiol Biomarkers Prev 3:675–682
Kalow W, Tang BK (1993) The use of caffeine for enzyme assays: a critical appraisal. Clin Pharmacol Ther 53:503–514
Kalow W, Tang BK (1991) Use of caffeine metabolite ratios to explore CYP1A2 and xanthine oxidase activities. Clin Pharmacol Ther 50:508–519
Denaro CP, Wilson M, Jacob III P, Benowitz NL (1996) The effect of liver disease on urine caffeine metabolic ratio. Clin Pharmacol Ther 59:624–635
Bechtel YC, Haffen E, Lelouet H, Brientini MP, Paintaud G, Miguet JP, Bechtel PR (2000) Relationship between the severity of alcoholic liver cirrhosis and the metabolism of caffeine in 226 patients. Int J Clin Pharmacol Ther 38:467–475
Bechtel YC, Lelouet H, Brientini MP, David-Laroche M, Miguet JP, Paintaud G, Bechtel PR (2000) Caffeine metabolism differences in acute hepatitis of viral and drug origin. Therapie 55:619–627
el-Yazigi A, Shabib S, al-Rawithi S, Yusuf A, Legayada ES, al-Humidan A (1999) Salivary clearance and urinary metabolic pattern of caffeine in healthy children and in pediatric patients with hepatocellular diseases. J Clin Pharmacol 39:366–372
Becquemont L, Chazouilleres O, Serfaty L, Poirier JM, Broly F, Jaillon P, Poupon R, Funck-Brentano C (2002) Effect of interferon alpha-ribavirin bitherapy on cytochrome P450 1A2 and 2D6 and N-acetyltransferase-2 activities in patients with chronic active hepatitis C. Clin Pharmacol Ther 71:488–495
Scott NR, Stambuk D, Chakraborty J, Marks V, Morgan MY (1988) Caffeine clearance and biotransformation in patients with chronic liver disease. Clin Sci 74:377–384
Fuhr U, Rost KL (1994) Simple and reliable CYP1A2 phenotyping by the paraxanthine/caffeine ratio in plasma and in saliva. Pharmacogenetics 4:109–116
Rostami-Hodjegan A, Nurminen S, Jackson PR, Tucker GT (1996) Caffeine urinary metabolite ratios as markers of enzyme activity: a theoretical assessment. Pharmacogenetics 6:121–149
Welfare MR, Bassendine MF, Daly AK (2000) The effect of NAT2 genotype and gender on the metabolism of caffeine in nonsmoking subjects. Br J Clin Pharmacol 49:240–243
Adedoyin A, Frye RF, Mauro K, Branch RA (1998) Chloroquine modulation of specific metabolizing enzymes activities: investigation with selective five drug cocktail. Br J Clin Pharmacol 46:215–219
Kukongviriyapan V, Prawan A, Tassaneyakul W, Aiemsa-Ard J, Warasiha B (2003) Arylamine N-acetyltransferase-2 genotypes in the Thai population. Br J Clin Pharmacol 55:278–281
Spigset O, Hagg S, Soderstrom E, Dahlqvist R (1999) The paraxanthine:caffeine ratio in serum or in saliva as a measure of CYP1A2 activity: when should the sample be obtained? Pharmacogenetics 9:409–412
Jost G, Wahllander A, von Mandach U, Preisig R (1987) Overnight salivary caffeine clearance: a liver function test suitable for routine use. Hepatology 7:338–344
Lewis FW, Rector WG Jr (1992) Caffeine clearance in cirrhosis. The value of simplified determinations of liver metabolic capacity. J Hepatol 14:157–162
Rizzo N, Hispard E, Dolbeault S, Dally S, Leverge R, Girre C (1997) Impact of long-term ethanol consumption on CYP1A2 activity. Clin Pharmacol Ther 62:505–509
Acknowledgements
This work was supported in part by the Faculty of Medicine Research Fund of Khon Kaen University and the National Research Council of Thailand. We thank Dr. Kanokwan Kittiwatanakul of Jitavej Khon Kaen Psychiatric Hospital for her advice and facilitation of the study. This study complies with the current Thai laws.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kukongviriyapan, V., Senggunprai, L., Prawan, A. et al. Salivary caffeine metabolic ratio in alcohol-dependent subjects. Eur J Clin Pharmacol 60, 103–107 (2004). https://doi.org/10.1007/s00228-004-0734-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-004-0734-3