Abstract
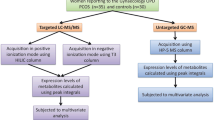
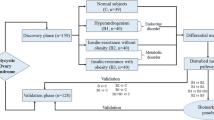
Metabolomics has become an important tool in distinguishing changes in metabolic pathways and the diagnosis of human disease. Polycystic ovary syndrome (PCOS) is a relatively complicated, heterogeneous endocrine disorder. The etiology and pathogenesis of PCOS remain uncertain. In this study, based on the platform of ultra performance liquid chromatography tandem quadrupole time-of-flight mass spectrometry (UPLC-QTOF-MS) and the method of pattern recognition, a comprehensive metabolomics approach has been applied to explore the changes in metabolic profiling between PCOS patients (n = 20) and controls (n = 15) as well as insulin-resistance (IR) PCOS patients (n = 11) and non-IR PCOS subjects (n = 9) in serum. In total, 36 metabolites were found significantly different between PCOS and controls, and 9 metabolites were discovered significantly different between IR and non-IR PCOS patients. Significant increases in the levels of saturated and unsaturated fatty acids (myristic acid, linoleic acid, 9-/13-HODE, etc.), fatty amides (palmitic amide, oleamide), dehydroepiandrosterone sulfate, l-glutamic acid, azelaic acid, l-glyceric acid, pyroglutamic acid, and decreases in the levels of lysophosphatidylethanolamine, lysophosphatidylcholine, uridine, and l-carnitine were found in PCOS patients compared with controls. In IR PCOS patients, linoleic acid, myristic acid, palmitoleic acid, and vaccenic acid also increased significantly compared with non-IR PCOS patients. All these changed metabolites showed abnormalities of steroid hormone biosynthesis, amino acids and nucleosides metabolism, glutathione metabolism, and lipids and carbohydrates metabolism in PCOS patients. The subgroup IR PCOS patients exhibited greater metabolic deviations than non-IR PCOS patients. These findings may help yield promising insights into the pathogenesis and advance the diagnosis and prevention of PCOS.

Serum metabolomics signature of polycystic ovary syndrome




Similar content being viewed by others
References
Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO (2004) The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 89:2745–2749
Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R (2011) Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol 7:219–231
Norman RJ, Dewailly D, Legro RS, Hickey TE (2007) Polycystic ovary syndrome. Lancet 370:685–697
Alvarez-Blasco F, Botella-Carretero JI, San Millan JL, Escobar-Morreale HF (2006) Prevalence and characteristics of the polycystic ovary syndrome in overweight and obese women. Arch Intern Med 166:2081–2086
Gonzalez F (2012) Inflammation in polycystic ovary syndrome: underpinning of insulin resistance and ovarian dysfunction. Steroids 77:300–305
Gambineri A, Patton L, Altieri P et al (2012) Polycystic ovary syndrome is a risk factor for type 2 diabetes: results from a long-term prospective study. Diabetes 61:2369–2374
Lo JC, Feigenbaum SL, Yang J, Pressman AR, Selby JV, Go AS (2006) Epidemiology and adverse cardiovascular risk profile of diagnosed polycystic ovary syndrome. J Clin Endocrinol Metab 91:1357–1363
Barry JA, Azizia MM, Hardiman PJ (2014) Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. doi:10.1093/humupd/dmu012
Hughes C, Elgasim M, Layfield R, Atiomo W (2006) Genomic and post-genomic approaches to polycystic ovary syndrome-progress so far: Mini Review. Hum Reprod 21:2766–2775
Diamanti-Kandarakis E, Piperi C, Spina J et al (2006) Polycystic ovary syndrome: the influence of environmental and genetic factors. Hormones 5:17–34
Diamanti-Kandarakis E, Dunaif A (2012) Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 33:981–1030
DeFronzo RA, Tobin JD, Andres R (1979) Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 6:E214–E223
Nicholson JK, Lindon JC, Holmes E (1999) ‘Metabonomics’: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 29:1181–1189
Nicholson JK, Lindon JC (2008) Systems biology: metabonomics. Nature 455:1054–1056
Newgard CB, An J, Bain FR et al (2009) A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 9:311–326
Wang-Sattler R, Yu Z, Herder C et al (2012) Novel biomarkers for pre-diabetes identified by metabolomics. Mol Syst Biol 8:615. doi:10.1038/msb.2012.43
Huang CF, Cheng ML, Fan CM, Hong CY, Shiao MS (2013) Nicotinuric acid: a potential marker of metabolic syndrome through a metabolomics-based approach. Diabetes Care 36:1729–1731
Mihalik SJ, Michaliszyn SF, Heras J et al (2012) Metabolomic profiling of fatty acid and amino acid metabolism in youth with obesity and type 2 diabetes. Diabetes Care 35:605–611
Atiomo W, Daykin CA (2012) Metabolomic biomarkers in women with polycystic ovary syndrome: a pilot study. Mol Hum Reprod 18:546–553
Sun L, Hu W, Liu Q et al (2012) Metabonomics reveals plasma metabolic changes and inflamatory marker in polycystic ovary syndrome patients. J Proteome Res 11:2937–2946
Zhao Y, Fu L, Li R et al (2012) Metabolic profiles characterizing different phenotypes of polycystic ovary syndrome: plasma metabolomics analysis. BMC Med 10:153. doi:10.1186/1741-7015-10-153
Escobar-Morreale HF, Samino S, Insenser M et al (2012) Metabolic heterogeneity in polycystic ovary syndrome is determined by obesity: plasma metabolomic approach using GC-MS. Clin Chem 58:999–1009
Zhang XJ, Huang LL, Su H et al (2014) Characterizing plasma phospholipid fatty acid profiles of polycystic ovary syndrome patients with and without insulin resistance using GC–MS and chemometrics approach. J Pharm Biomed Anal 95:85–92
Zhao YY, Cheng XL, Wei F et al (2012) Serum metabonomics study of adenine-induced chronic renal failure in rats by ultra performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. Biomarkers 17:48–55
Zhao Y, Xu F, Qi B et al (2014) Serum metabolomics study of polycystic ovary syndrome based on liquid chromatography−mass spectrometry. J Proteome Res 13:1101–1111
Rotterdam E, P ASRM-Sponsored (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19:41–47
Yang S, Li Q, Song Y et al (2011) Serum complement C3 has a stronger association with insulin resistance than high-sensitivity C-reactive protein in women with polycystic ovary syndrome. Fertil Steril 95:1749–1753
Bijlsma S, Bobeldijk L, Verheij ER, Ramaker R, Kochhar S, Macdonald IA, van Ommen B, Smilde AK (2006) Large-scale human metabolomics studies: a strategy for data (pre-) processing and validation. Anal Chem 78:567–574
Westerhuis JA, van Velzen EJJ, Hoefsloot HCJ, Smilde AK (2010) Multivariate paired data analysis: multilevel PLSDA versus OPLSDA. Metabolomics 6:119–128
Young SP, Kapoor SR, Viant MR, Byrne JJ, Filer A et al (2013) The impact of inflammation on metabolomic profiles in patients with arthritis. Arthritis Rheum 65:2015–2023
Boelaert J, t'Kindt R, Schepers E et al (2014) State-of-the-art non-targeted metabolomics in the study of chronic kidney disease. Metabolomics 10:425–442
Abraham GE (1974) Ovarian and adrenal contribution to peripheral androgens during the menstrual cycle. J Clin Endocrinol Metab 39:340–346
Legro RS, Kunselman AR, Demers L, Wang SC, Bentley-Lewis R, Dunaif A (2002) Elevated dehydroepiandrosterone sulfate levels as the reproductive phenotype in the brothers of women with polycystic ovary syndrome. J Clin Endocrinol Metab 87:2134–2138
Stener-Victorin E, Holm G, Labrie F, Nilsson L, Janson PO, Ohlsson C (2010) Are there any sensitive and specific sex steroid markers for polycystic ovary syndrome? J Clin Endocrinol Metab 95:810–819
Exton JH (1994) Phosphatidylcholine breakdown and signal transduction. Biochim Biophys Acta 1212:26–42
Murakami I, Wakasa Y, Yamashita S, Kurihara T, Zama K, Kobayashi N, Mizutani Y, Mitsutake S, Shigyo T, Igarashi Y (2011) Phytoceramide and sphingoid bases derived from brewer's yeast Saccharomyces pastorianus activate peroxisome proliferator-activated receptors. Lipids Health Dis 10:150. doi:10.1186/1476-511X-10-150
Murakami I, Mitsutake S, Kobayashi N, Matsuda J, Suzuki A, Shigyo T, Igarashi Y (2013) Improved high-fat diet-induced glucose intolerance by an oral administration of phytosphingosine. Biosci Biotechnol Biochem 77:194–197
Snel M, Sleddering MA, Pijl H, Nieuwenhuizen WF, Frölich M, Havekes LM, Romijn JA, Jazet IM (2010) The effect of dietary phytosphingosine on cholesterol levels and insulin sensitivity in subjects with the metabolic syndrome. Eur J Clin Nutr 64:419–423
Gillett MP, Obineche EN, Lakhani MS et al (2001) Levels of cholesteryl esters and other lipids in the plasma of patients with end-stage renal failure. Ann Saudi Med 21:283–286
Floegel A, Stefan N, Yu ZH et al (2013) Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 62:639–648
Liu L, Wang M, Yang X et al (2013) Fasting serum lipid and dehydroepiandrosterone sulfate as important metabolites for detecting isolated postchallenge diabetes: serum metabolomics via ultra-high-performance liquid chromatography/mass spectrometry. Clin Chem 59:1338–1348
Muoio DM (2012) Revisiting the connection between intramyocellular lipids and insulin resistance: a long and winding road. Diabetologia 55:2551–2554
Tushuizen ME, Bunck MC, Pouwels PJ et al (2007) Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care 30:2916–2921
Vinaixa M, Rodriguez MA, Samino S et al (2011) Metabolomics reveals reduction of metabolic oxidation in women with polycystic ovary syndrome after pioglitazone-flutamide-metformin polytherapy. PLoS One 6:e29052
Harizi H, Corcuff JB, Gualde N (2008) Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology. Trends Mol Med 14:461–469
Marzo VD (2008) The endocannabinoid system in obesity and type 2 diabetes. Diabetologia 51:1356–1367
Fenkci SM, Fenkci V, Oztekin O, Rota S, Karagenc N (2008) Serum total L-carnitine levels in non-obese women with polycystic ovary syndrome. Hum Reprod 23:1602–1606
Dunning KR, Robker RL (2012) Promoting lipid utilization with L-carnitine to improve oocyte quality. Anim Reprod Sci 134:69–75
Zhang C, Zhao Y, Li R et al (2014) Metabolic heterogeneity of follicular amino acids in polycystic ovary syndrome is affected by obesity and related to pregnancy outcome. BMC Pregnancy Childbirth 14:11. doi:10.1186/1471-2393-14-11
Newgard CB (2012) Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab 15:606–614
Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O’Donnell CJ, Carr SA, Mootha VK, Flore JC, Souza A, Melander O, Clish CB, Gerszten RE (2011) Metabolite profiles and the risk of developing diabetes. Nat Med 17:448–453
Acknowledgments
This work was supported by the National Key Clinical Specialties Construction Program of China (Grant No. 2011 (170)), the Natural Science Foundation Project of CQ (Grant No. CSTC2013jjB10019), and the National Natural Science Foundation of China (Grant No. 81371904). The present study was completed successfully with the help of Medical Examination Centre & Department of Endocrinology of The First Affiliated Hospital of Chongqing Medical University, and Department of Life Science of Chongqing Medical University.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Fang Dong and Dan Deng contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 148 kb)
Rights and permissions
About this article
Cite this article
Dong, F., Deng, D., Chen, H. et al. Serum metabolomics study of polycystic ovary syndrome based on UPLC-QTOF-MS coupled with a pattern recognition approach. Anal Bioanal Chem 407, 4683–4695 (2015). https://doi.org/10.1007/s00216-015-8670-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8670-x