Abstract
Rationale
Criteria for remission in schizophrenia have recently been presented. It is unclear how many acutely ill patients meet these criteria and how they compare with previously suggested definitions.
Objectives and methods
We re-analysed seven anti-psychotic drug trials (n = 1,708) of patients with schizophrenia to find out how many met the new remission criteria and their single components, how many met two previously used remission criteria, and how many met simpler measures of response (at least 50% Brief Psychiatric Rating Scale [BPRS] reduction, a Clinical Global Impressions [CGI] improvement score of at least ‘much better’ or a CGI severity score of ‘mild or better’).
Results
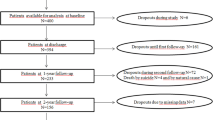
Thirty-seven percent/41% (last observation carried forward [LOCF]/completer analysis [CO]) of the initially acutely ill patients with positive symptoms met the severity criteria of remission at 4 weeks, and 27%/52% (worst case/CO) met the severity and time criteria at 1 year. Only 13%/21% (LOCF) met the severity criteria at 4 weeks/1 year when an item threshold ‘at best very mild symptoms’ was applied, and almost no patients were absolutely symptom-free. The psychotic symptoms component was more difficult to achieve than the negative component. The criteria were more stringent than ‘at least 50% BPRS reduction’ and than ‘CGI improvement score of at least much better.’ However, the definition ‘CGI severity score mild or better’ was of a stringency similar to the new remission criteria, which probably explains why fewer patients met previously defined criteria that included this scale.
Conclusion
The new remission criteria proved to be an achievable goal for clinical trials. A consensus on the application of their time component is still needed.




Similar content being viewed by others
References
American Psychiatric Association (1987) Diagnostic and statistical manual for mental disorders, third revision, revised (DSM-III-R). American Psychiatric Association, Washington, DC
American Psychiatric Association (1994) Diagnostic and statistical manual for mental disorders, 4th edn. American Psychiatric Association, Washington, DC
Andreasen NC (1989) Scale for the assessment of negative symptoms. Br J Psychiatry 155(Suppl 7):53–58
Andreasen N, Carpenter W, Kane J, Lasser R, Marder S, Weinberger D (2005) Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry 62:441–449
Arvanitis LA, Miller BG, Seroquel Trial 13 Study Group (1997) Multiple fixed doses of “seroquel” (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. Biol Psychiatry 42:233–246
Beasley CM, Tollefson GD, Tran P, Satterlee W, Sanger T, Hamilton S, Olanzapine HGAD Study Group (1996) Olanzapine versus haloperidol and placebo. Acute phase results of the american double-blind olanzapine trial. Neuropsychopharmacology 14:111–123
Boyer P, Lecrubier Y, Puech AJ, Dewailly J, Aubin F (1995) Treatment of negative symptoms in schizophrenia with amisulpride. Br J Psychiatry 166:68–72
Carrière P, Bonhomme D, Lempérière T (2000) Amisulpride has superior benefit: risk profile to haloperidol in schizophrenia: results of a multicentre, double-blind study (the Amisulpride study group). Eur Psychiatr 15:321–329
Colonna L, Saleem P, Dondey-Nouvel L, Rein W, Amisulpride Study Group (2000) Long-term safety and efficacy of amisulpride in subchronic or chronic schizophrenia. Int Clin Psychopharmacol 15:13–22
Costa e Silva JA (1989) Comparative double-blind study of amisulpride versus haloperidol in the treatment of acute psychotic states. Amisulpride. Expansion Scientifique Francaise, Paris, pp 93–104
Danion JM, Rein W, Fleurot O (1999) Improvement of schizophrenic patients with primary negative symptoms treated with amisulpride. Am J Psychiatry 156:610–616
De Hert M, van Winkel R, Wampers M, Kane J, van Os J, Peuskens J (2007) Remission criteria for schizophrenia: evaluation in a large naturalistic cohort. Schizophr Res 92:68–73
Delcker A, Schoon ML, Oczkowski B, Gaertner HJ (1990) Amisulpride versus haloperidol in treatment of schizophrenic patients- results of a double-blind study. Pharmacopsychiatry 23:125–130
Docherty JP, Bossle CA, Lachaux B, Bouhours P, Zhu Y, Lasser R, Gharabawi GM (2007) Patient-based and clinician-based support for the remission criteria in schizophrenia. Int Clin Psychopharmacol 22:51–55
Doyle AC, Pollack MH (2003) Establishment of remission criteria for anxiety disorders. J Clin Psychiatry 64:40–45
Emsley R, Rabinowitz J, Medori R, Early Psychosis Global Working Group (2007) Remission in early psychosis: rates, predictors, and clinical and functional outcome correlates. Schizophr Res 89:129–139
Guy W (1976) Clinical Global Impressions. ECDEU assessment manual for psychopharmacology, revised (DHEW Publ. no. ADM 76-338). National Institute of Mental Health, Rockville, MD, pp 218–222
Harding CM, Brooks GW, Ashikaga T, Strauss JS, Breier A (1987) The Vermont longitudinal-study of persons with severe mental-illness. 1. Methodology, study sample, and overall status 32 years later. Am J Psychiatr 144:718–726
Haro JM, Kamath SA, Ochoa S, Novick D, Rele K, Fargas A, Rodriguez MJ, Rele R, Orta J, Kharbeng A, Araya S, Gervin M, Alonso J, Mavreas V, Lavrentzou E, Liontos N, Gregor K, Jones PB (2003) The Clinical Global Impression—Schizophrenia scale: a simple instrument to measure the diversity of symptoms present in schizophrenia. Acta Psychiatr Scand 107:16–23
Haro JM, Edgell ET, Novick D, Alonso J, Kennedy L, Jones PB, Ratcliffe M, Breier A (2005) Effectiveness of antipsychotic treatment for schizophrenia: 6-month results of the Pan-European Schizophrenia Outpatient Health Outcomes (SOHO) study. Acta Psychiatr Scand 111:220–231
Haro JM, Ochoa S, Gervin M, Mavreas V, Jones P (2007) Assessment of remission in schizophrenia with the CGI and CGI-SCH scales. Acta Psychiatr Scand 115:163–164
Harrison G, Hopper K, Craig T, Laska E, Siegel C, Wanderling J, Dube KC, Ganev K, Giel R, An der Heiden W, Holmberg SK, Janca A, Lee PWH, Leon CA, Malhotra S, Marsella AJ, Nakane Y, Sartorius N, Shen Y, Skoda C, Thara R, Tsirkin SJ, Varma VK, Walsh D, Wiersma D (2001) Recovery from psychotic illness: a 15- and 25-year international follow-up study. Br J Psychiatry 178:506–517
Helldin L, Kane JM, Karilampi U, Norlander T, Archer T (2006) Remission and cognitive ability in a cohort of patients with schizophrenia. J Psychiatr Res 40:738–745
Helldin L, Kane JM, Karilampi U, Norlander T, Archer T (2007) Remission in prognosis of functional outcome: a new dimension in the treatment of patients with psychotic disorders. Schizophr Res 93:160–168
Huber G, Gross G, Schuttler R, Linz M (1980) Longitudinal-studies of schizophrenic-patients. Schizophr Bull 6:592–605
Kane JM, Honigfeld G, Singer J, Meltzer H, Clozaril Collaborative Study Group (1988) Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry 45:789–796
Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–275
Kissling S, Heres S, Lloyd K, Sacchetti E, Bouhours P, Medori R, Llorca PM (2005) Direct transition to long-acting risperidone- analysis of long-term efficacy. J Psychopharmacol 19:15–21
Klein HE, Dieterle D, Rüther E, Eben E, Nedopil N, Hippius H (1985) A double-blind comparison of amisulpride vs. haloperidol in acute schizophrenic patients. In: Pichot P, Berner P, Wolf R, Thau K (eds) Psychiatry, the state of the art. Plenum, New York, pp 687–691
Lasser RA, Bossie CA, Gharabawi GM, Kane JM (2005) Remission in schizophrenia: results from a 1-year study of long-acting risperidone injection. Schizophr Res 77:215–227
Leucht S, Engel RR (2006) The relative sensitivity of the clinical global impressions scale and the brief psychiatric rating scale in antipsychotic drug trials. Neuropsychopharmacology 31:406–412
Leucht S, Lasser R (2006) The concepts of remission and recovery in schizophrenia. Pharmacopsychiatry 39:161–170
Leucht S, Pitschel-Walz G, Engel R, Kissling W (2002) Amisulpride—an unusual atypical antipsychotic. A meta-analysis of randomized controlled trials. Am J Psychiatry 159:180–190
Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR (2005a) Clinical implications of BPRS scores. Br J Psychiatry 187:363–371
Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR (2005b) What does the PANSS mean? Schizophr Res 79:231–238
Leucht S, Kane JM, Etschel E, Hamann J, Kissling W, Engel RR (2006) Linking the PANSS, BPRS and CGI. Clinical implications. Neuropsychopharmacology 3:2318–2325
Liberman R, Kopelowicz A, Ventura J, Gutkind D (2002) Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry 14:256–272
Lieberman JA, Phillips M, Gu H, Stroup S, Zhang P, Kong L, Ji ZKG, Hamer RM (2003) Atypical and conventional antipsychotic drugs in treatment-naive first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology 28:995–1003
Marder SR, Meibach RC (1994) Risperidone in the treatment of schizophrenia. Am J Psychiatry 151:825–835
Möller HJ, Boyer P, Fleurot O, Rein W (1997) Improvement of acute exacerbations of schizophrenia with amisulpride: a comparison with haloperidol. Psychopharmacology 132:396–401
Mortimer A, Martin S, Loo H, Peuskens J (2004) A double-blind, randomized comparative trial of amisulpride versus olanzapine for 6 months in the treatment of schizophrenia. Int Clin Psychopharmacol 19:63–69
Overall JE, Gorham DR (1962) The brief psychiatric rating scale. Psychol Rep 10:790–812
Peuskens J, Link CGG (1997) A comparison of quetiapine and chlorpromazine in the treatment of schizophrenia. Acta Psychiatr Scand 96:265–273
Peuskens J, Bech P, Möller HJ, Bale R, Fleurot O, Rein W, Amisulpride Study Group (1999) Amisulpride vs. risperidone in the treatment of acute exacerbations of schizophrenia. Psychiatry Res 88:107–117
Pichot P, Boyer P (1988) Etude multicentrique controlée en double insu: amisulpride (Solian 200) versus halopéridol à forte dose dans les états psychotiques aigus. Ann Psychiatr 3:326–332
Puech A, Fleurot O, Rein W (1998) Amisulpride, an atypical antipsychotic, in the treatment of acute episodes of schizophrenia: a dose-ranging study vs. haloperidol. Acta Psychiatr Scand 98:65–72
Sechter D, Peuskens J, Fleurot O, Rein W, Lecrubier Y (2002) Amisulpride vs. risperidone in chronic schizophrenia: results of a 6-month double-blind study. Neuropsychopharmacology 27:1071–1081
Sethuraman G, Taylor CC, Enerson M, Dunayevich E (2005) A retrospective comparison of cumulative time spent in remission during treatment with olanzapine or risperidone among patients with schizophrenia. Schizophr Res 79:337–340
Small JG, Hirsch SR, Arvanitis LA, Miller BG, Link CGG, Seroquel Study Group (1997) Quetiapine in patients with schizophrenia. A high- and low-dose comparison with placebo. Arch Gen Psychiatry 54:549–557
Speller JC, Barnes TRE, Curson DA, Pantelis C, Alberts JL (1997) One-year, low-dose neuroleptic study of in-patients with chronic schizophrenia characterised by persistent negative symptoms—amisulpride vs. haloperidol. Br J Psychiatry 171:564–568
van Os J, Burns T, Cavallaro R, Leucht S, Peuskens J, Helldin L, Bernardo M, Arango C, Fleischhacker W, Lachaux B, Kane JM (2006a) Standardized remission criteria in schizophrenia. Acta Psychiatr Scand 113:91–95
van Os J, Drukker M, Campo JA, Meijer J, Bak M, Delespaul P (2006b) Validation of remission criteria for schizophrenia. Am J Psychiatry 163:2000–2001
Wahlbeck K, Tuunainen A, Ahokas A, Leucht S (2001) Drop-out rates in randomised antipsychotic drug trials. Psychopharmacology 155:230–233
Wetzel H, Grunder G, Hillert A, Philipp M, Gattaz WF, Sauer H, Adler G, Schroder J, Rein W, Benkert O (1998) Amisulpride versus flupentixol in schizophrenia with predominantly positive symptomatology—a double-blind controlled study comparing a selective D-2-like antagonist to a mixed D-1-/D-2-like antagonist. Psychopharmacology 137:223–232
Ziegler B (1989) Study of the efficacy of a substituted benzamide amisulpride, versus haloperidol, in productive schizophrenia. Amisulpride. Expansion Scientifique Francaise, Paris, pp 73–81
Acknowledgements
We are indebted to SanofiAventis for allowing us to analyse individual patient data from their databases. The study was supported by the American Psychiatric Association/AstraZeneca Young Minds in Psychiatry Award 2004.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material
Supplement 1
(DOC 205 KB)
Rights and permissions
About this article
Cite this article
Leucht, S., Beitinger, R. & Kissling, W. On the concept of remission in schizophrenia. Psychopharmacology 194, 453–461 (2007). https://doi.org/10.1007/s00213-007-0857-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-007-0857-1