Abstract
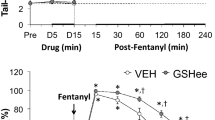
Fentanyl-induced hyperalgesia and antinociception after systemic administration has been shown in previous clinical and experimental studies. However, there is very little evidence regarding the local possible effects of fentanyl. The purpose of this study was to assess whether local (intraplantar) fentanyl administration can produce antinociception and hyperalgesia. In addition, we examined the effects of magnesium, N-methyl-D-aspartate receptor antagonist, on possible changes produced by fentanyl. The paw withdrawal latencies to radiant heat stimuli were measured to assess the thermal nociceptive actions. Intraplantar administration of fentanyl caused time and dose-dependent increase in the paw withdrawal latencies (antinociception). Coinjection of magnesium with fentanyl markedly enhanced the antinociception. However, fentanyl also markedly decreased paw withdrawal latencies 24 h after intraplantar administration (hyperalgesia). In the presence of magnesium, hyperalgesia after fentanyl administration was not observed. Consequently, following the fentanyl administration, local hyperalgesia after antinociception is a negative effect in pain treatment. Magnesium may not only prevent the hyperalgesia but also enhance antinociceptive effect of fentanyl.



Similar content being viewed by others
References
Angst MS, Clark JD (2006) Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 104:570–587
Antonijevic I, Mousa SA, Schafer M, Stein C (1995) Perineurial defect and peripheral opioid analgesia in inflammation. J Neurosci 15(l):165–172
Begon S, Pickering G, Eschalier A, Dubray C (2000) Magnesium and MK-801 have a similar effect in two experimental models of neuropathic pain. Brain Res 887:436–439
Begon S, Pickering G, Eschalier A, Dubray C (2002) Magnesium ıncreases morphine analgesic effect in different experimental models of pain. Anesthesiology 96:627–632
Celerier E, Rivat C, Jun Y, Laulin JP, Larcher A, Reynier P, Simonnet G (2000) Long-lasting hyperalgesia induced by fentanyl in rats: preventive effect of ketamine. Anesthesiology 92:465–472
Celerier E, Laulin JP, Corcuff JB, Le Moal M, Simonnet G (2001) Progressive enhancement of delayed hyperalgesia induced by repeated heroin administration: a sensitization process. J Neurosci 21:4074–4080
Chen L, Huang LY (1991) Sustained potentiation of NMDA receptor-mediated glutamate responses through activation of protein kinase C by a mu opioid. Neuron 7:319–326
Dunbar SA, Pulai IJ (1998) Repetitive opioid abstinence causes progressive hyperalgesia sensitive to N-methyl-D-aspartate receptor blockade in the rat. J Pharmacol Exp Ther 284:678–686
DuPen A, Shen D, Ersek M (2007) Mechanisms of opioid-induced tolerance and hyperalgesia. Pain Manag Nurs 8(3):113–121
Janson W, Stein C (2003) Peripheral opioid analgesia. Curr Pharm Biotechnol 4:270–274
Laulin JP, Maurette P, Corcuff JB, Rivat C, Chauvin M, Simonnet G (2002) The role of ketamine in preventing fentanyl-induced hyperalgesia and subsequent acute morphine tolerance. Anesth Analg 94:1263–1269
Mao J (2002) Opioid-induced abnormal pain sensitivity: implications in clinical opioid therapy. Pain 100:213–217
Martin TJ, Eisenach JC (2001) Pharmacology of opioid and nonopioid analgesics in chronic pain states. J Pharmacol Exp Ther 299:811–817
Mert T, Gunes Y, Gunay I (2007) Local analgesic efficacy of tramadol following intraplantar injection. Eur J Pharmacol 558:68–72
Millan MJ (1999) The induction of pain: an integrative review. Prog Neurobiol 57:1–164
Obara I, Przewlocki R, Przewlocka B (2004) Local peripheral effects of mu-opioid receptor agonists in neuropathic pain in rats. Neurosci Lett 360:85–89
Petrenko AB, Yamakura T, Baba H, Shimoji K (2003) The role of N-Methyl-D-Aspartate (NMDA) receptors in pain: a review. Anesth Analg 97:1108–1116
Rivat C, Laulin JP, Corcuff JB, Celerier E, Pain L, Simonnet G (2002) Fentanyl enhancement of carrageenan-induced long-lasting hyperalgesia in rats: prevention by the N-methyl-D-aspartate receptor antagonist ketamine. Anesthesiology 96:381–391
Sawynok J (2003) Topical and peripherally acting analgesics. Pharmacol Rev 55:1–20
Simonnet G, Rivat C (2003) Opioid-induced hyperalgesia: abnormal or normal pain? NeuroReport 14:1–6
Stein C, Machelska H, Binder W, Schafer M (2001) Peripheral opioid analgesia. Curr Opin Pharmacol 1:62–65
Sukiennik AW, Kream RM (1995) N-methyl-D-aspartate receptors and pain. Curr Opin Anaesthesiol 8:445–449
Takano Y, Sato E, Kaneko T, Sato I (2000) Antihyperalgesic effects of intrathecally administered magnesium sulfate in rats. Pain 84:175–179
Todorovic SM, Jevtovic-Todorovic V, Meyenburg A, Mennerick S, Perez-Reyes E, Romano C, Olney JW, Zorumski CF (2001) Redox modulation of T-type calcium channels in rat peripheral nociceptors. Neuron 31(1):75–85
Truong W, Cheng C, Xu QG, Li XQ, Zochodne DW (2003) Mu opioid receptors and analgesia at the site of a peripheral nerve injury. Ann Neurol 53:366–375
Van Elstraete AC, Sitbon P, Mazoit JX, Conti M, Benhamou D (2006) Protective effect of prior administration of magnesium on delayed hyperalgesia induced by fentanyl in rats. Can J Anesth 53(12):1180–1185
Acknowledgments
We acknowledge the support given by the Cukurova University Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mert, T., Gunes, Y., Ozcengiz, D. et al. Magnesium modifies fentanyl-induced local antinociception and hyperalgesia. Naunyn-Schmied Arch Pharmacol 380, 415–420 (2009). https://doi.org/10.1007/s00210-009-0447-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-009-0447-3