Abstract
5F-MDMB-PINACA and 4F-MDMB-BINACA are synthetic cannabinoids (SCs) that elicit cannabinoid psychoactive effects. Defining pharmacokinetic–pharmacodynamic (PK–PD) relationships governing SCs and their metabolites are paramount to investigating their in vivo toxicological outcomes. However, the disposition kinetics and cannabinoid receptor (CB) activities of the primary metabolites of SCs are largely unknown. Additionally, reasons underlying the selection of ester hydrolysis metabolites (EHMs) as urinary biomarkers are often unclear. Here, metabolic reaction phenotyping was performed to identify key metabolizing enzymes of the parent SCs. Hepatic clearances of parent SCs and their EHMs were estimated from microsomal metabolic stability studies. Renal clearances were simulated using a mechanistic kidney model incorporating in vitro permeability and organic anionic transporter 3 (OAT3)-mediated uptake data. Overall clearances were considered in tandem with estimated volumes of distribution for in vivo biological half-lives (t1/2) predictions. Interactions of the compounds with CB1 and CB2 were investigated using a G-protein coupled receptor activation assay. We demonstrated that similar enzymatic isoforms were implicated in the metabolism of 5F-MDMB-PINACA and 4F-MDMB-BINACA. Our in vivo t1/2 determinations verified the rapid elimination of parent SCs and suggest prolonged circulation of their EHMs. The pronounced attenuation of the potencies and efficacies of the metabolites against CB1 and CB2 further suggests how toxic manifestations of SC abuse are likely precipitated by augmented exposure to parent SCs. Notably, basolateral OAT3-mediated uptake of the EHMs substantiates their higher urinary abundance. These novel insights underscore the importance of mechanistic, quantitative and systematic characterization of PK–PD relationships in rationalizing the toxicities of SCs.





Similar content being viewed by others
Abbreviations
- AUCm/AUCp :
-
Relative area under the curve of metabolite to parent
- BNPP:
-
Bis(4-nitrophenyl) phosphate
- CB:
-
Cannabinoid receptor
- C b/C :
-
Ratio of drug concentration in blood to plasma
- CLbsl,scr :
-
In vivo basolateral secretion clearance
- CLformation :
-
In vivo formation clearance of the ester hydrolysis metabolite
- CLH :
-
In vivo hepatic clearance
- CLint,active :
-
In vitro intrinsic transporter-mediated active uptake
- CLint,passive :
-
In vitro intrinsic passive uptake
- CLint,formation :
-
In vitro intrinsic formation clearance of the ester hydrolysis metabolite
- CLint,metabolic :
-
In vitro intrinsic metabolic clearance of the ester hydrolysis metabolite
- CLint,total :
-
In vitro total intrinsic clearance of the parent synthetic cannabinoid
- CLR :
-
In vivo renal clearance
- CYP450:
-
Cytochrome P450 enzyme
- CES:
-
Carboxylesterase
- DDI:
-
Drug–drug interaction
- EC50 :
-
Concentration of agonist that provokes a response halfway between the baseline and maximum response
- EHM:
-
Ester hydrolysis metabolite
- E max :
-
The maximal limit of response to an agonist
- f u :
-
Fraction unbound of drug in plasma
- f ub :
-
Fraction unbound of drug in blood
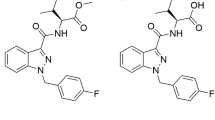
- 4F-MDMB-BINACA:
-
Methyl 2‐[1-(4‐fluorobutyl)‐1H‐indazole‐3‐carboxamido]‐3,3-dimethylbutanoate
- 5F-MDMB-PINACA:
-
Methyl 2‐[1-(5‐fluoropentyl)‐1H‐indazole‐3‐carboxamido]‐3,3‐dimethylbutanoate
- HEK:
-
Human embryonic kidney 293
- HLM:
-
Human liver microsomes
- ISEF:
-
Intersystem extrapolation factor
- IVIVE:
-
In vitro in vivo extrapolation
- k :
-
Elimination rate constant
- MDCK II:
-
Madin–Darby Canine Kidney II
- nsSNP:
-
Non-synonymous small nucleotide polymorphism
- OAT:
-
Organic anion transporter
- P app :
-
Apparent permeability
- pK a :
-
Acid dissociation constant
- rhCYP450:
-
Recombinant human cytochrome P450 enzyme
- rhCES:
-
Recombinant human carboxylesterase
- SC:
-
Synthetic cannabinoid
- t 1/2 :
-
Half-life
- UGT:
-
Uridine 5′-diphospho-glucuronosyltransferase
- USFDA:
-
United States Food and Drug Administration
- V ss :
-
Volume of distribution at steady state
References
Antonides LH, Cannaert A, Norman C et al (2019) Enantiospecific synthesis, chiral separation, and biological activity of four indazole-3-carboxamide-type synthetic cannabinoid receptor agonists and their detection in seized drug samples. Front Chem 7:1–20
Cannaert A, Storme J, Franz F et al (2016) Detection and activity profiling of synthetic cannabinoids and metabolites with a newly developed bio-assay. Anal Chem 88:11476–11485
Cannaert A, Franz F, Auwärter V, Stove CP (2017) Activity-based detection of consumption of synthetic cannabinoids in authentic urine samples using a stable cannabinoid reporter system. Anal Chem 89:9527–9536
Castaneto MS, Wohlfarth A, Desrosiers NA et al (2015) Synthetic cannabinoids pharmacokinetics and detection methods in biological matrices. Drug Metab Rev 47:124–174
Chen Y, Liu L, Nguyen K, Fretland AJ (2011) Utility of intersystem extrapolation factors in early reaction phenotyping and the quantitative extrapolation of human liver microsomal intrinsic clearance using recombinant cytochromes P450. Drug Metab Dispos 39:373–382
Diao X, Huestis MA (2017) Approaches, challenges, and advances in metabolism of new synthetic cannabinoids and identification of optimal urinary marker metabolites. Clin Pharmacol Ther 101:239–253
Diao X, Huestis MA (2019) New synthetic cannabinoids metabolism and strategies to best identify optimal marker metabolites. Front Chem 7:109
European Monitoring Centre for Drugs and Drug Addiction (2017) 5F-MDMB-PINACA—Report on the risk assessment of methyl 2-{[1-(5-fluoropentyl)-1H-indazole-3-carbonyl]amino}-3,3-dimethylbutanoate in the framework of the Council Decision on new psychoactive substances
Gamage TF, Farquhar CE, McKinnie RJ et al (2019) Synthetic cannabinoid hydroxypentyl metabolites retain efficacy at human cannabinoid receptors. J Pharmacol Exp Ther 368:414–422
Gatch MB, Forster MJ (2019) Cannabinoid-like effects of five novel carboxamide synthetic cannabinoids. Neurotoxicology 70:72–79
Gurney SMR, Scott KS, Kacinko SL et al (2014) Pharmacology, toxicology, and adverse effects of synthetic cannabinoid drugs. Forensic Sci Rev 26:54–76
Harris CR, Brown A (2013) Synthetic cannabinoid intoxication: a case series and review. J Emerg Med 44:360–366
Haschimi B, Mogler L, Halter S et al (2019) Detection of the recently emerged synthetic cannabinoid 4F-MDMB-BINACA in ‘legal high’ products and human urine specimens. Drug Test Anal 11:1377–1386
Holm NB, Nielsen LM, Linnet K (2015a) CYP3A4 mediates oxidative metabolism of the synthetic cannabinoid AKB-48. AAPS J 17:1237–1245
Holm NB, Pedersen AJ, Dalsgaard PW, Linnet K (2015b) Metabolites of 5F-AKB-48, a synthetic cannabinoid receptor agonist, identified in human urine and liver microsomal preparations using liquid chromatography high-resolution mass spectrometry. Drug Test Anal 7:199–206
Huang W, Isoherranen N (2018) Development of a dynamic physiologically based mechanistic kidney model to predict renal clearance. CPT Pharmacometrics Syst Pharmacol 7:593–602
Huestis MA, Gorelick DA, Heishman SJ et al (2001) Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry 58:322–328
Hussaarts KGAM, Veerman GDM, Jansman FGA et al (2019) Clinically relevant drug interactions with multikinase inhibitors: a review. Ther Adv Med Oncol 11:1–34
Izumi S, Nozaki Y, Kusuhara H et al (2018) Relative activity factor (raf)-based scaling of uptake clearance mediated by organic anion transporting polypeptide (OATP) 1B1 and OATP1B3 in human hepatocytes. Mol Pharm 15:2277–2288
Kong TY, Kim JH, Kim DK, Lee HS (2018) Synthetic cannabinoids are substrates and inhibitors of multiple drug-metabolizing enzymes. Arch Pharm Res 41:691–710
Krotulski AJ, Mohr AL, Logan BK (2018) Trend Report: Q4 2018 Synthetic Cannabinoids in the United States
Krotulski AJ, Mohr AL, Kacinko SL et al (2019) 4F-MDMB-BINACA: a new synthetic cannabinoid widely implicated in forensic casework. J Forensic Sci 64:1451–1461
Mathialagan S, Piotrowski MA, Tess DA et al (2017) Quantitative prediction of human renal clearance and drug-drug interactions of organic anion transporter substrates using in vitro transport data: a relative activity factor approach. Drug Metab Dispos 45:409–417
Meyer MR, Schütz A, Maurer HH (2015) Contribution of human esterases to the metabolism of selected drugs of abuse. Toxicol Lett 232:159–166
Nguyen HQ, Lin J, Kimoto E et al (2017) Prediction of losartan-active carboxylic acid metabolite exposure following losartan administration using static and physiologically based pharmacokinetic models. J Pharm Sci 106:2758–2770
Noble C, Cannaert A, Linnet K, Stove CP (2019) Application of an activity-based receptor bioassay to investigate the in vitro activity of selected indole- and indazole-3-carboxamide-based synthetic cannabinoids at CB1 and CB2 receptors. Drug Test Anal 11:501–511
Obach RS (1999) Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: an examination of in vitro half-life approach and nonspecific binding to microsomes. Drug Metab Dispos 27:1350–1359
Poulin P, Theil FP (2002) Prediction of pharmacokinetics prior to in vivo studies. 1. Mechanism-based prediction of volume of distribution. J Pharm Sci 91:129–156
Prakash C, Kamel A, Cui D et al (2000) Identification of the major human liver cytochrome P450 isoform(s) responsible for the formation of the primary metabolites of ziprasidone and prediction of possible drug interactions. Br J Clin Pharmacol 49:35–42
Presley BC, Jansen-Varnum SA, Logan BK (2013) Analysis of synthetic cannabinoids in botanical material: a review of analytical methods and findings. Forensic Sci Rev 25:27–46
Reidy L, Seither J, Boland D (2018) Identification of synthetic cannabinoid 5-fluoro-ADB in human performance and postmortem samples: a case series. J Forensic Toxicol Pharmacol 7:1–9
Riley RJ, McGinnity DF, Austin RP (2005) A unified model for predicting human hepatic, metabolic clearance from in vitro intrinsic clearance data in hepatocytes and microsomes. Drug Metab Dispos 33:1304–1311
Rodgers T, Rowland M (2006) Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci 95:1238–1257. https://doi.org/10.1002/jps.20502
Seither JZ, Reidy LJ, Boland DM (2020) Identification and quantification of 5-fluoro ADB and the 5-fluoro ADB ester hydrolysis metabolite in postmortem blood samples by LC-MS/MS. J Anal Toxicol 44:133–139
Tai S, Fantegrossi WE (2016) Pharmacological and toxicological effects of synthetic cannabinoids and their metabolites. In: Current topics in behavioral neurosciences. Springer Verlag, pp 249–262
Thomsen R, Nielsen LM, Holm NB et al (2015) Synthetic cannabimimetic agents metabolized by carboxylesterases. Drug Test Anal 7:565–576
Tran TT, Mittal A, Gales T et al (2004) Exact kinetic analysis of passive transport across a polarized confluent MDCK cell monolayer modeled as a single barrier. J Pharm Sci 93:2108–2123
United Nations Office on Drugs and Crime (2019) Current NPS Threats, vol 1
U.S. Food and Drug Administration (2017) Drug Development and Drug Interactions: Table of Substrates, Inhibitors and Inducers. https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers. Accessed 18 Aug 2019
US FDA (2017) Drug Development and Drug Interactions: Table of Substrates, Inhibitors and Inducers
Wang X, Rida N, Shi J et al (2017) A comprehensive functional assessment of carboxylesterase 1 nonsynonymous polymorphisms. Drug Metab Dispos 45:1149–1155
Wang D, Zou L, Jin Q et al (2018) Human carboxylesterases: a comprehensive review. Acta Pharm Sin B 8:699–712
Wouters E, Mogler L, Cannaert A et al (2019) Functional evaluation of carboxy metabolites of synthetic cannabinoid receptor agonists featuring scaffolds based on L-valine or L-tert-leucine. Drug Test Anal 11:1183–1191
Yanjiao X, Chengliang Z, Xiping L et al (2013) Evaluation of the inhibitory effects of antihypertensive drugs on human carboxylesterase in vitro. Drug Metab Pharmacokinet 28:468–474
Yeter O, Öztürk YE (2019) Detection and quantification of 5F-ADB and its methyl ester hydrolysis metabolite in fatal intoxication cases by liquid chromatography–high resolution mass spectrometry. Forensic Sci Int 302:109866
Yeter O, Ozturk YE (2019) Metabolic profiling of synthetic cannabinoid 5F-ADB by human liver microsome incubations and urine samples using high-resolution mass spectrometry. Drug Test Anal 11:847–858
Zhao A, Tan M, Maung A et al (2015) Rhabdomyolysis and acute kidney injury requiring dialysis as a result of concomitant use of atypical neuroleptics and synthetic cannabinoids. Case Reports Nephrol 2015:1–4
Acknowledgements
The authors thank Jye Ing Soah, Yen Li Tan and Ching Yee Fong for their assistance in the LC-MS/MS instrumentation. The authors also thank Chi Pang Lui for his feedback. The project is supported by the Singapore Health Sciences Authority (HSA) and the National University of Singapore (NUS) Department of Pharmacy’s Final Year Project (FYP) funding provided to E.C.Y.C. A. C. acknowledges funding as a postdoctoral research fellow from the Research Foundation-Flanders (FWO; 12Y9520N). C. S. acknowledges funding from the Ghent University—Special Research Fund (Grants no. 01N00814 and no. 01J15517).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lie, W., Cheong, E.J.Y., Goh, E.M.L. et al. Diagnosing intake and rationalizing toxicities associated with 5F-MDMB-PINACA and 4F-MDMB-BINACA abuse. Arch Toxicol 95, 489–508 (2021). https://doi.org/10.1007/s00204-020-02948-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-020-02948-3