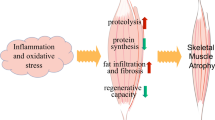
Abstract
With advancing age, the skeletal muscle phenotype is characterized by a progressive loss of mass, strength, and quality. This phenomenon, known as sarcopenia, has a negative impact on quality of life and increases the risk of morbidity and mortality in older adults. Accumulating evidence suggests that damaged and dysfunctional mitochondria play a critical role in the pathogenesis of sarcopenia. Lifestyle modifications, such as physical activity, exercise, and nutrition, as well as medical interventions with therapeutic agents, are effective in the management of sarcopenia and offer solutions to maintain and improve skeletal muscle health. Although a great deal of effort has been devoted to the identification of the best treatment option, these strategies are not sufficient to overcome sarcopenia. Recently, it has been reported that mitochondrial transplantation may be a possible therapeutic approach for the treatment of mitochondria-related pathological conditions such as ischemia, liver toxicity, kidney injury, cancer, and non-alcoholic fatty liver disease. Given the role of mitochondria in the function and metabolism of skeletal muscle, mitochondrial transplantation may be a possible option for the treatment of sarcopenia. In this review, we summarize the definition and characteristics of sarcopenia and molecular mechanisms associated with mitochondria that are known to contribute to sarcopenia. We also discuss mitochondrial transplantation as a possible option. Despite the progress made in the field of mitochondrial transplantation, further studies are needed to elucidate the role of mitochondrial transplantation in sarcopenia.
Key messages
-
Sarcopenia is the progressive loss of skeletal muscle mass, strength, and quality.
-
Although the specific mechanisms that lead to sarcopenia are not fully understood, mitochondria have been identified as a key factor in the development of sarcopenia.
-
Damaged and dysfunctional mitochondria initiate various cellular mediators and signaling pathways, which largely contribute to the age-related loss of skeletal muscle mass and strength.
-
Mitochondrial transplantation has been reported to be a possible option for the treatment/prevention of several diseases.
-
Mitochondrial transplantation may be a possible therapeutic option for improving skeletal muscle health and treating sarcopenia.
Graphical Abstract

Mitochondrial transplantation as a possible treatment option for sarcopenia.




Similar content being viewed by others
References
Luo J, Mills K, le Cessie S, Noordam R, van Heemst D (2020) Ageing, age-related diseases and oxidative stress: what to do next? Ageing Res Rev 57:100982. https://doi.org/10.1016/j.arr.2019.100982
Tosato M, Zamboni V, Ferrini A, Cesari M (2007) The aging process and potential interventions to extend life expectancy. Clin Interv Aging 2:401–412
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153:1194–1217. https://doi.org/10.1016/j.cell.2013.05.039
Cesari M, Landi F, Vellas B, Bernabei R, Marzetti E (2014) Sarcopenia and physical frailty: two sides of the same coin. Front Aging Neurosci 6:192. https://doi.org/10.3389/fnagi.2014.00192
Rebelo-Marques A, De Sousa LA, Andrade R, Ribeiro CF, Mota-Pinto A, Carrilho F, Espregueira-Mendes J (2018) Aging hallmarks: the benefits of physical exercise. Front Endocrinol (Lausanne) 9:258. https://doi.org/10.3389/fendo.2018.00258
Schmauck-Medina T, Moliere A, Lautrup S, Zhang J, Chlopicki S, Madsen HB, Cao S, Soendenbroe C, Mansell E, Vestergaard MB et al (2022) New hallmarks of ageing: a 2022 Copenhagen ageing meeting summary. Aging (Albany NY) 14:6829–6839. https://doi.org/10.18632/aging.204248
McCay CM, Crowell MF, Maynard LA (1935) The effect of retarded growth upon the length of life span and upon the ultimate body size: one figure. J Nutr 10:63–79
Crimmins EM (2015) Lifespan and healthspan: past, present, and promise. Gerontologist 55:901–911. https://doi.org/10.1093/geront/gnv130
Kaeberlein M, Rabinovitch PS, Martin GM (2015) Healthy aging: the ultimate preventative medicine. Science 350:1191–1193. https://doi.org/10.1126/science.aad3267
Seo DY, Lee SR, Kim N, Ko KS, Rhee BD, Han J (2016) Age-related changes in skeletal muscle mitochondria: the role of exercise. Integr Med Res 5:182–186. https://doi.org/10.1016/j.imr.2016.07.003
Lee SY, Tung HH, Liu CY, Chen LK (2018) Physical activity and sarcopenia in the geriatric population: a systematic review. J Am Med Dir Assoc 19:378–383. https://doi.org/10.1016/j.jamda.2018.02.003
Zhang Y, Hao Q, Ge M, Dong B (2018) Association of sarcopenia and fractures in community-dwelling older adults: a systematic review and meta-analysis of cohort studies. Osteoporos Int 29:1253–1262. https://doi.org/10.1007/s00198-018-4429-5
Naruse M, Fountain WA, Claiborne A, Finch WH, Trappe S, Trappe TA (2023) Muscle group-specific skeletal muscle aging: a 5-yr longitudinal study in septuagenarians. J Appl Physiol (1985) 134:915–922. https://doi.org/10.1152/japplphysiol.00769.2022
Wiedmer P, Jung T, Castro JP, Pomatto LCD, Sun PY, Davies KJA, Grune T (2021) Sarcopenia - molecular mechanisms and open questions. Ageing Res Rev 65:101200. https://doi.org/10.1016/j.arr.2020.101200
Bowen TS, Schuler G, Adams V (2015) Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle 6:197–207. https://doi.org/10.1002/jcsm.12043
Marzetti E, Calvani R, Cesari M, Buford TW, Lorenzi M, Behnke BJ, Leeuwenburgh C (2013) Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int J Biochem Cell Biol 45:2288–2301. https://doi.org/10.1016/j.biocel.2013.06.024
Coen PM, Musci RV, Hinkley JM, Miller BF (2018) Mitochondria as a target for mitigating sarcopenia. Front Physiol 9:1883. https://doi.org/10.3389/fphys.2018.01883
Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS (2005) Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA 102:5618–5623. https://doi.org/10.1073/pnas.0501559102
Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI (2003) Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300:1140–1142. https://doi.org/10.1126/science.1082889
Tyynismaa H, Mjosund KP, Wanrooij S, Lappalainen I, Ylikallio E, Jalanko A, Spelbrink JN, Paetau A, Suomalainen A (2005) Mutant mitochondrial helicase Twinkle causes multiple mtDNA deletions and a late-onset mitochondrial disease in mice. Proc Natl Acad Sci USA 102:17687–17692. https://doi.org/10.1073/pnas.0505551102
Bellanti F, Lo Buglio A, Vendemiale G (2021) Mitochondrial impairment in sarcopenia. Biology (Basel) 10. https://doi.org/10.3390/biology10010031
Gouspillou G, Sgarioto N, Kapchinsky S, Purves-Smith F, Norris B, Pion CH, Barbat-Artigas S, Lemieux F, Taivassalo T, Morais JA et al (2014) Increased sensitivity to mitochondrial permeability transition and myonuclear translocation of endonuclease G in atrophied muscle of physically active older humans. FASEB J 28:1621–1633. https://doi.org/10.1096/fj.13-242750
Roubenoff R, Hughes VA (2000) Sarcopenia: current concepts. J Gerontol A Biol Sci Med Sci 55:M716-724. https://doi.org/10.1093/gerona/55.12.m716
Doherty TJ (2003) Invited review: Aging and sarcopenia. J Appl Physiol (1985) 95:1717–1727. https://doi.org/10.1152/japplphysiol.00347.2003
Hood DA, Memme JM, Oliveira AN, Triolo M (2019) Maintenance of skeletal muscle mitochondria in health, exercise, and aging. Annu Rev Physiol 81:19–41. https://doi.org/10.1146/annurev-physiol-020518-114310
Ganapathy A, Nieves JW (2020) Nutrition and sarcopenia-what do we know? Nutrients 12. https://doi.org/10.3390/nu12061755
Wu PY, Huang KS, Chen KM, Chou CP, Tu YK (2021) Exercise, nutrition, and combined exercise and nutrition in older adults with sarcopenia: a systematic review and network meta-analysis. Maturitas 145:38–48. https://doi.org/10.1016/j.maturitas.2020.12.009
Smith C, Woessner MN, Sim M, Levinger I (2022) Sarcopenia definition: does it really matter? Implications for resistance training. Ageing Res Rev 78:101617. https://doi.org/10.1016/j.arr.2022.101617
Wang H, Huang WY, Zhao Y (2022) Efficacy of exercise on muscle function and physical performance in older adults with sarcopenia: an updated systematic review and meta-analysis. Int J Environ Res Public Health 19. https://doi.org/10.3390/ijerph19138212
Beaudart C, Dawson A, Shaw SC, Harvey NC, Kanis JA, Binkley N, Reginster JY, Chapurlat R, Chan DC, Bruyere O et al (2017) Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int 28:1817–1833. https://doi.org/10.1007/s00198-017-3980-9
Cannataro R, Carbone L, Petro JL, Cione E, Vargas S, Angulo H, Forero DA, Odriozola-Martinez A, Kreider RB, Bonilla DA (2021) Sarcopenia:etiology, nutritional approaches, and miRNAs. Int J Mol Sci 22. https://doi.org/10.3390/ijms22189724
Trouwborst I, Verreijen A, Memelink R, Massanet P, Boirie Y, Weijs P, Tieland M (2018) Exercise and nutrition strategies to counteract sarcopenic obesity. Nutrients 10. https://doi.org/10.3390/nu10050605
Hawley JA, Hargreaves M, Joyner MJ, Zierath JR (2014) Integrative biology of exercise. Cell 159:738–749. https://doi.org/10.1016/j.cell.2014.10.029
McGee SL, Hargreaves M (2020) Exercise adaptations: molecular mechanisms and potential targets for therapeutic benefit. Nat Rev Endocrinol 16:495–505. https://doi.org/10.1038/s41574-020-0377-1
Moreira JBN, Wohlwend M, Wisloff U (2020) Exercise and cardiac health: physiological and molecular insights. Nat Metab 2:829–839. https://doi.org/10.1038/s42255-020-0262-1
Ziaaldini MM, Marzetti E, Picca A, Murlasits Z (2017) Biochemical pathways of sarcopenia and their modulation by physical exercise: a narrative review. Front Med (Lausanne) 4:167. https://doi.org/10.3389/fmed.2017.00167
Ulger O, Kubat GB (2022) Therapeutic applications of mitochondrial transplantation. Biochimie 195:1–15. https://doi.org/10.1016/j.biochi.2022.01.002
McCully JD, Levitsky S, Del Nido PJ, Cowan DB (2016) Mitochondrial transplantation for therapeutic use. Clin Transl Med 5:16. https://doi.org/10.1186/s40169-016-0095-4
Gollihue JL, Rabchevsky AG (2017) Prospects for therapeutic mitochondrial transplantation. Mitochondrion 35:70–79. https://doi.org/10.1016/j.mito.2017.05.007
Zhang Z, Yan C, Miao J, Pu K, Ma H, Wang Q (2021) Muscle-derived mitochondrial transplantation reduces inflammation, enhances bacterial clearance, and improves survival in sepsis. Shock 56:108–118. https://doi.org/10.1097/SHK.0000000000001681
Lee JM, Hwang JW, Kim MJ, Jung SY, Kim KS, Ahn EH, Min K, Choi YS (2021) Mitochondrial transplantation modulates inflammation and apoptosis, alleviating tendinopathy both in vivo and in vitro. Antioxidants (Basel) 10. https://doi.org/10.3390/antiox10050696
Orfany A, Arriola CG, Doulamis IP, Guariento A, Ramirez-Barbieri G, Moskowitzova K, Shin B, Blitzer D, Rogers C, Del Nido PJ et al (2020) Mitochondrial transplantation ameliorates acute limb ischemia. J Vasc Surg 71:1014–1026. https://doi.org/10.1016/j.jvs.2019.03.079
Masuzawa A, Black KM, Pacak CA, Ericsson M, Barnett RJ, Drumm C, Seth P, Bloch DB, Levitsky S, Cowan DB et al (2013) Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 304:H966-982. https://doi.org/10.1152/ajpheart.00883.2012
McCully JD, Cowan DB, Pacak CA, Toumpoulis IK, Dayalan H, Levitsky S (2009) Injection of isolated mitochondria during early reperfusion for cardioprotection. Am J Physiol Heart Circ Physiol 296:H94–H105. https://doi.org/10.1152/ajpheart.00567.2008
Hayashida K, Takegawa R, Shoaib M, Aoki T, Choudhary RC, Kuschner CE, Nishikimi M, Miyara SJ, Rolston DM, Guevara S et al (2021) Mitochondrial transplantation therapy for ischemia reperfusion injury: a systematic review of animal and human studies. J Transl Med 19:214. https://doi.org/10.1186/s12967-021-02878-3
Ulger O, Kubat GB, Cicek Z, Celik E, Atalay O, Suvay S, Ozler M (2021) The effects of mitochondrial transplantation in acetaminophen-induced liver toxicity in rats. Life Sci 279: 119669. https://doi.org/10.1016/j.lfs.2021.119669
Kubat GB, Ozler M, Ulger O, Ekinci O, Atalay O, Celik E, Safali M, Budak MT (2021) The effects of mesenchymal stem cell mitochondrial transplantation on doxorubicin-mediated nephrotoxicity in rats. J Biochem Mol Toxicol 35:e22612. https://doi.org/10.1002/jbt.22612
Jabbari H, Roushandeh AM, Rostami MK, Razavi-Toosi MT, Shokrgozar MA, Jahanian-Najafabadi A, Kuwahara Y, Roudkenar MH (2020) Mitochondrial transplantation ameliorates ischemia/reperfusion-induced kidney injury in rat. Biochim Biophys Acta Mol Basis Dis 1866:165809. https://doi.org/10.1016/j.bbadis.2020.165809
Chang JC, Chang HS, Wu YC, Cheng WL, Lin TT, Chang HJ, Kuo SJ, Chen ST, Liu CS (2019) Mitochondrial transplantation regulates antitumour activity, chemoresistance and mitochondrial dynamics in breast cancer. J Exp Clin Cancer Res 38:30. https://doi.org/10.1186/s13046-019-1028-z
Yan C, Ma Z, Ma H, Li Q, Zhai Q, Jiang T, Zhang Z, Wang Q (2020) Mitochondrial transplantation attenuates brain dysfunction in sepsis by driving microglial M2 polarization. Mol Neurobiol 57:3875–3890. https://doi.org/10.1007/s12035-020-01994-3
Fu A, Shi X, Zhang H, Fu B (2017) Mitotherapy for fatty liver by intravenous administration of exogenous mitochondria in male mice. Front Pharmacol 8:241. https://doi.org/10.3389/fphar.2017.00241
Kim MJ, Hwang JW, Yun C-K, Lee Y, Choi Y-S (2018) Delivery of exogenous mitochondria via centrifugation enhances cellular metabolic function. Sci Rep 8:3330. https://doi.org/10.1038/s41598-018-21539-y
Kim MJ, Lee JM, Min K, Choi YS (2023) Xenogeneic transplantation of mitochondria induces muscle regeneration in an in vivo rat model of dexamethasone-induced atrophy. J Muscle Res Cell Motil. https://doi.org/10.1007/s10974-023-09643-7. Advance online publication. https://doi.org/10.1007/s10974-023-09643-7
Alway SE, Paez HG, Pitzer CR, Ferrandi PJ, Khan MM, Mohamed JS, Carson JA, Deschenes MR (2023) Mitochondria transplant therapy improves regeneration and restoration of injured skeletal muscle. J Cachexia Sarcopenia Muscle. https://doi.org/10.1002/jcsm.13153
Rosenberg IH (1997) Sarcopenia: origins and clinical relevance. J Nutr 127:990S-991S. https://doi.org/10.1093/jn/127.5.990S
Metter EJ, Conwit R, Tobin J, Fozard JL (1997) Age-associated loss of power and strength in the upper extremities in women and men. J Gerontol A Biol Sci Med Sci 52:B267-276. https://doi.org/10.1093/gerona/52a.5.b267
Narici MV, Maffulli N (2010) Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull 95:139–159. https://doi.org/10.1093/bmb/ldq008
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48:16–31. https://doi.org/10.1093/ageing/afy169
Rolland Y, Lauwers-Cances V, Cristini C, van Kan GA, Janssen I, Morley JE, Vellas B (2009) Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: the EPIDOS (EPIDemiologie de l’OSteoporose) Study. Am J Clin Nutr 89:1895–1900
Dominguez LJ, Barbagallo M (2007) The cardiometabolic syndrome and sarcopenic obesity in older persons. J Cardiometab Syndr 2:183–189
Cruz-Jentoft AJ, Landi F, Schneider SM, Zuniga C, Arai H, Boirie Y, Chen LK, Fielding RA, Martin FC, Michel JP et al (2014) Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 43:748–759. https://doi.org/10.1093/ageing/afu115
Petermann-Rocha F, Balntzi V, Gray SR, Lara J, Ho FK, Pell JP, Celis-Morales C (2022) Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 13:86–99. https://doi.org/10.1002/jcsm.12783
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM et al (2010) Sarcopenia:european consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age Ageing 39:412–423. https://doi.org/10.1093/ageing/afq034
Cade WT, Yarasheski KE (2006) Metabolic and molecular aspects of sarcopenia. Principles of Molecular Medicine Springer, pp. 529–534
Lexell J, Taylor CC, Sjöström M (1988) What is the cause of the ageing atrophy?: Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15-to 83-year-old men. J Neurol Sci 84:275–294
Pedersen BK, Febbraio MA (2012) Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 8:457–465. https://doi.org/10.1038/nrendo.2012.49
Janssen I, Heymsfield SB, Wang ZM (1985) Ross R (2000) Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol 89:81–88. https://doi.org/10.1152/jappl.2000.89.1.81
Fluck M, Hoppeler H (2003) Molecular basis of skeletal muscle plasticity–from gene to form and function. Rev Physiol Biochem Pharmacol 146:159–216. https://doi.org/10.1007/s10254-002-0004-7
Frontera WR, Ochala J (2015) Skeletal muscle: a brief review of structure and function. Calcif Tissue Int 96:183–195. https://doi.org/10.1007/s00223-014-9915-y
Chow LS, Gerszten RE, Taylor JM, Pedersen BK, van Praag H, Trappe S, Febbraio MA, Galis ZS, Gao Y, Haus JM et al (2022) Exerkines in health, resilience and disease. Nat Rev Endocrinol 18:273–289. https://doi.org/10.1038/s41574-022-00641-2
Sabaratnam R, Wojtaszewski JFP, Hojlund K (2022) Factors mediating exercise-induced organ crosstalk. Acta Physiol (Oxf) 234:e13766. https://doi.org/10.1111/apha.13766
Magliulo L, Bondi D, Pini N, Marramiero L, Di Filippo ES (2022) The wonder exerkines-novel insights: a critical state-of-the-art review. Mol Cell Biochem 477:105–113. https://doi.org/10.1007/s11010-021-04264-5
Turkel I, Ozerklig B, Atakan MM, Aktitiz S, Kosar SN, Yazgan B (2022) Exercise and metabolic health: the emerging roles of novel exerkines. Curr Protein Pept Sci 23:437–455. https://doi.org/10.2174/1389203723666220629163524
Sartori R, Romanello V, Sandri M (2021) Mechanisms of muscle atrophy and hypertrophy: implications in health and disease. Nat Commun 12:330. https://doi.org/10.1038/s41467-020-20123-1
Amorim JA, Coppotelli G, Rolo AP, Palmeira CM, Ross JM, Sinclair DA (2022) Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat Rev Endocrinol 18:243–258. https://doi.org/10.1038/s41574-021-00626-7
McBride HM, Neuspiel M, Wasiak S (2006) Mitochondria: more than just a powerhouse. Curr Biol 16:R551-560. https://doi.org/10.1016/j.cub.2006.06.054
Kim SJ, Xiao J, Wan J, Cohen P, Yen K (2017) Mitochondrially derived peptides as novel regulators of metabolism. J Physiol 595:6613–6621. https://doi.org/10.1113/JP274472
Roger AJ, Munoz-Gomez SA, Kamikawa R (2017) The origin and diversification of mitochondria. Curr Biol 27:R1177–R1192. https://doi.org/10.1016/j.cub.2017.09.015
Ferreira R, Vitorino R, Alves RM, Appell HJ, Powers SK, Duarte JA, Amado F (2010) Subsarcolemmal and intermyofibrillar mitochondria proteome differences disclose functional specializations in skeletal muscle. Proteomics 10:3142–3154. https://doi.org/10.1002/pmic.201000173
Cogswell AM, Stevens RJ, Hood DA (1993) Properties of skeletal muscle mitochondria isolated from subsarcolemmal and intermyofibrillar regions. Am J Physiol 264:C383-389. https://doi.org/10.1152/ajpcell.1993.264.2.C383
Adhihetty PJ, Ljubicic V, Menzies KJ, Hood DA (2005) Differential susceptibility of subsarcolemmal and intermyofibrillar mitochondria to apoptotic stimuli. Am J Physiol Cell Physiol 289:C994–C1001. https://doi.org/10.1152/ajpcell.00031.2005
Picard M, White K (1985) Turnbull DM (2013) Mitochondrial morphology, topology, and membrane interactions in skeletal muscle: a quantitative three-dimensional electron microscopy study. J Appl Physiol 114:161–171. https://doi.org/10.1152/japplphysiol.01096.2012
Koves TR, Noland RC, Bates AL, Henes ST, Muoio DM, Cortright RN (2005) Subsarcolemmal and intermyofibrillar mitochondria play distinct roles in regulating skeletal muscle fatty acid metabolism. Am J Physiol Cell Physiol 288:C1074-1082. https://doi.org/10.1152/ajpcell.00391.2004
Takahashi M, Hood DA (1996) Protein import into subsarcolemmal and intermyofibrillar skeletal muscle mitochondria. Differential import regulation in distinct subcellular regions. J Biol Chem 271:27285–27291. https://doi.org/10.1074/jbc.271.44.27285
Chabi B, Ljubicic V, Menzies KJ, Huang JH, Saleem A, Hood DA (2008) Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell 7:2–12. https://doi.org/10.1111/j.1474-9726.2007.00347.x
Alway SE, Mohamed JS, Myers MJ (2017) Mitochondria initiate and regulate sarcopenia. Exerc Sport Sci Rev 45:58–69. https://doi.org/10.1249/JES.0000000000000101
Conley KE, Jubrias SA, Esselman PC (2000) Oxidative capacity and ageing in human muscle. J Physiol 526(Pt 1):203–210. https://doi.org/10.1111/j.1469-7793.2000.t01-1-00203.x
Tian Q, Mitchell BA, Zampino M, Fishbein KW, Spencer RG, Ferrucci L (2022) Muscle mitochondrial energetics predicts mobility decline in well-functioning older adults: the baltimore longitudinal study of aging. Aging Cell 21:e13552. https://doi.org/10.1111/acel.13552
Carter HN, Chen CC, Hood DA (2015) Mitochondria, muscle health, and exercise with advancing age. Physiology (Bethesda) 30:208–223. https://doi.org/10.1152/physiol.00039.2014
Kang C, Chung E, Diffee G, Ji LL (2013) Exercise training attenuates aging-associated mitochondrial dysfunction in rat skeletal muscle: role of PGC-1alpha. Exp Gerontol 48:1343–1350. https://doi.org/10.1016/j.exger.2013.08.004
Pesce V, Cormio A, Fracasso F, Lezza AM, Cantatore P, Gadaleta MN (2005) Age-related changes of mitochondrial DNA content and mitochondrial genotypic and phenotypic alterations in rat hind-limb skeletal muscles. J Gerontol A Biol Sci Med Sci 60:715–723. https://doi.org/10.1093/gerona/60.6.715
Koltai E, Hart N, Taylor AW, Goto S, Ngo JK, Davies KJ, Radak Z (2012) Age-associated declines in mitochondrial biogenesis and protein quality control factors are minimized by exercise training. Am J Physiol Regul Integr Comp Physiol 303:R127-134. https://doi.org/10.1152/ajpregu.00337.2011
Ferri E, Marzetti E, Calvani R, Picca A, Cesari M, Arosio B (2020) Role of age-related mitochondrial dysfunction in sarcopenia. Int J Mol Sci 21. https://doi.org/10.3390/ijms21155236
Buffenstein R (2005) The naked mole-rat: a new long-living model for human aging research. J Gerontol A Biol Sci Med Sci 60:1369–1377. https://doi.org/10.1093/gerona/60.11.1369
Stoll EA, Karapavlovic N, Rosa H, Woodmass M, Rygiel K, White K, Turnbull DM, Faulkes CG (2016) Naked mole-rats maintain healthy skeletal muscle and Complex IV mitochondrial enzyme function into old age. Aging (Albany NY) 8:3468–3485. https://doi.org/10.18632/aging.101140
Inci N, Kamali D, Akyildiz EO, Tahir Turanli E, Bozaykut P (2022) Translation of cellular senescence to novel therapeutics: insights from alternative tools and models. Front Aging 3:828058. https://doi.org/10.3389/fragi.2022.828058
Tait SW, Green DR (2012) Mitochondria and cell signalling. J Cell Sci 125:807–815. https://doi.org/10.1242/jcs.099234
Powers SK, Goldstein E, Schrager M, Ji LL (2023) Exercise training and skeletal muscle antioxidant enzymes: an update. Antioxidants 12:39
Brennan LA, Kantorow M (2009) Mitochondrial function and redox control in the aging eye: role of MsrA and other repair systems in cataract and macular degenerations. Exp Eye Res 88:195–203. https://doi.org/10.1016/j.exer.2008.05.018
Zorov DB, Juhaszova M, Sollott SJ (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94:909–950. https://doi.org/10.1152/physrev.00026.2013
Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95. https://doi.org/10.1152/physrev.00018.2001
Powers SK, Kavazis AN, DeRuisseau KC (2005) Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol 288:R337-344. https://doi.org/10.1152/ajpregu.00469.2004
Andreyev AY, Kushnareva YE, Starkov AA (2005) Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 70:200–214. https://doi.org/10.1007/s10541-005-0102-7
Fulle S, Protasi F, Di Tano G, Pietrangelo T, Beltramin A, Boncompagni S, Vecchiet L, Fanò G (2004) The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp Gerontol 39:17–24. https://doi.org/10.1016/j.exger.2003.09.012
Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK (2018) Reactive oxygen species in metabolic and inflammatory signaling. Circ Res 122:877–902. https://doi.org/10.1161/CIRCRESAHA.117.311401
Baumann CW, Kwak D, Liu HM, Thompson LV (2016) Age-induced oxidative stress: how does it influence skeletal muscle quantity and quality? J Appl Physiol 121:1047–1052. https://doi.org/10.1152/japplphysiol.00321.2016
Carmeli E, Coleman R, Reznick AZ (2002) The biochemistry of aging muscle. Exp Gerontol 37:477–489. https://doi.org/10.1016/S0531-5565(01)00220-0
Mecocci P, Fano G, Fulle S, MacGarvey U, Shinobu L, Polidori MC, Cherubini A, Vecchiet J, Senin U, Beal MF (1999) Age-dependent increases in oxidative damage to DNA, lipids, and proteins in human skeletal muscle. Free Radic Biol Med 26:303–308. https://doi.org/10.1016/s0891-5849(98)00208-1
Howard C, Ferrucci L, Sun K, Fried LP, Walston J, Varadhan R, Guralnik JM (1985) Semba RD (2007) Oxidative protein damage is associated with poor grip strength among older women living in the community. J Appl Physiol 103:17–20. https://doi.org/10.1152/japplphysiol.00133.2007
Hepple RT, Hagen JL, Krause DJ (1985) Jackson CC (2003) Aerobic power declines with aging in rat skeletal muscles perfused at matched convective O2 delivery. J Appl Physiol 94:744–751. https://doi.org/10.1152/japplphysiol.00737.2002
Campbell MD, Duan J, Samuelson AT, Gaffrey MJ, Merrihew GE, Egertson JD, Wang L, Bammler TK, Moore RJ, White CC et al (2019) Improving mitochondrial function with SS-31 reverses age-related redox stress and improves exercise tolerance in aged mice. Free Radic Biol Med 134:268–281. https://doi.org/10.1016/j.freeradbiomed.2018.12.031
Siegel MP, Kruse SE, Percival JM, Goh J, White CC, Hopkins HC, Kavanagh TJ, Szeto HH, Rabinovitch PS, Marcinek DJ (2013) Mitochondrial-targeted peptide rapidly improves mitochondrial energetics and skeletal muscle performance in aged mice. Aging Cell 12:763–771. https://doi.org/10.1111/acel.12102
Sakellariou GK, Pearson T, Lightfoot AP, Nye GA, Wells N, Giakoumaki II, Vasilaki A, Griffiths RD, Jackson MJ, McArdle A (2016) Mitochondrial ROS regulate oxidative damage and mitophagy but not age-related muscle fiber atrophy. Sci Rep 6:33944. https://doi.org/10.1038/srep33944
Gianni P, Jan KJ, Douglas MJ, Stuart PM, Tarnopolsky MA (2004) Oxidative stress and the mitochondrial theory of aging in human skeletal muscle. Exp Gerontol 39:1391–1400. https://doi.org/10.1016/j.exger.2004.06.002
Capel F, Rimbert V, Lioger D, Diot A, Rousset P, Mirand PP, Boirie Y, Morio B, Mosoni L (2005) Due to reverse electron transfer, mitochondrial H2O2 release increases with age in human vastus lateralis muscle although oxidative capacity is preserved. Mech Ageing Dev 126:505–511. https://doi.org/10.1016/j.mad.2004.11.001
Powers SK, Hudson MB, Nelson WB, Talbert EE, Min K, Szeto HH, Kavazis AN, Smuder AJ (2011) Mitochondrial-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit Care Med 39:1749
Min K, Smuder AJ, Kwon O-s, Kavazis AN, Szeto HH, Powers SK (2011) Mitochondrial-targeted antioxidants protect skeletal muscle against immobilization-induced muscle atrophy. J Appl Physiol 111:1459–1466
Urso ML, Clarkson PM (2003) Oxidative stress, exercise, and antioxidant supplementation. Toxicology 189:41–54
Kanter MM (1994) Free radicals, exercise, and antioxidant supplementation. Int J Sport Nutr Exerc Metab 4:205–220
Antuna E, Cachan-Vega C, Bermejo-Millo JC, Potes Y, Caballero B, Vega-Naredo I, Coto-Montes A, Garcia-Gonzalez C (2022) Inflammaging: implications in sarcopenia. Int J Mol Sci 23. https://doi.org/10.3390/ijms232315039
Bano G, Trevisan C, Carraro S, Solmi M, Luchini C, Stubbs B, Manzato E, Sergi G, Veronese N (2017) Inflammation and sarcopenia: a systematic review and meta-analysis. Maturitas 96:10–15. https://doi.org/10.1016/j.maturitas.2016.11.006
Livshits G, Kalinkovich A (2019) Inflammaging as a common ground for the development and maintenance of sarcopenia, obesity, cardiomyopathy and dysbiosis. Ageing Res Rev 56: 100980. https://doi.org/10.1016/j.arr.2019.100980
Wang T (2022) Searching for the link between inflammaging and sarcopenia. Ageing Res Rev 77:101611. https://doi.org/10.1016/j.arr.2022.101611
Lopez-Armada MJ, Riveiro-Naveira RR, Vaamonde-Garcia C, Valcarcel-Ares MN (2013) Mitochondrial dysfunction and the inflammatory response. Mitochondrion 13:106–118. https://doi.org/10.1016/j.mito.2013.01.003
Cadenas E, Davies KJ (2000) Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29:222–230. https://doi.org/10.1016/s0891-5849(00)00317-8
Thannickal VJ, Fanburg BL (2000) Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279:L1005-1028. https://doi.org/10.1152/ajplung.2000.279.6.L1005
Nian M, Lee P, Khaper N, Liu P (2004) Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res 94:1543–1553. https://doi.org/10.1161/01.RES.0000130526.20854.fa
Martinez de Toda I, Ceprian N, Diaz-Del Cerro E, De la Fuente M (2021) The role of immune cells in oxi-inflamm-aging. Cells 10. https://doi.org/10.3390/cells10112974
Tuttle CSL, Thang LAN, Maier AB (2020) Markers of inflammation and their association with muscle strength and mass: a systematic review and meta-analysis. Ageing Res Rev 64:101185. https://doi.org/10.1016/j.arr.2020.101185
Dalle S, Rossmeislova L, Koppo K (2017) The role of inflammation in age-related sarcopenia. Front Physiol 8:1045. https://doi.org/10.3389/fphys.2017.01045
Schaap LA, Pluijm SM, Deeg DJ, Visser M (2006) Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med 119(526):e529–e517. https://doi.org/10.1016/j.amjmed.2005.10.049
Baker RG, Hayden MS, Ghosh S (2011) NF-kappaB, inflammation, and metabolic disease. Cell Metab 13:11–22. https://doi.org/10.1016/j.cmet.2010.12.008
Serrano AL, Baeza-Raja B, Perdiguero E, Jardi M, Munoz-Canoves P (2008) Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab 7:33–44. https://doi.org/10.1016/j.cmet.2007.11.011
Li YP, Chen Y, John J, Moylan J, Jin B, Mann DL, Reid MB (2005) TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J 19:362–370. https://doi.org/10.1096/fj.04-2364com
Xia Z, Cholewa J, Zhao Y, Shang HY, Yang YQ, Araujo Pessoa K, Su QS, Lima-Soares F, Zanchi NE (2017) Targeting inflammation and downstream protein metabolism in sarcopenia: a brief up-dated description of concurrent exercise and leucine-based multimodal intervention. Front Physiol 8:434. https://doi.org/10.3389/fphys.2017.00434
Sciorati C, Gamberale R, Monno A, Citterio L, Lanzani C, De Lorenzo R, Ramirez GA, Esposito A, Manunta P, Manfredi AA et al (2020) Pharmacological blockade of TNFalpha prevents sarcopenia and prolongs survival in aging mice. Aging (Albany NY) 12:23497–23508. https://doi.org/10.18632/aging.202200
Quiles JM, Gustafsson AB (2020) Mitochondrial quality control and cellular proteostasis: two sides of the same coin. Front Physiol 11:515. https://doi.org/10.3389/fphys.2020.00515
Tatsuta T, Langer T (2008) Quality control of mitochondria: protection against neurodegeneration and ageing. EMBO J 27:306–314. https://doi.org/10.1038/sj.emboj.7601972
Romanello V, Sandri M (2015) Mitochondrial quality control and muscle mass maintenance. Front Physiol 6:422. https://doi.org/10.3389/fphys.2015.00422
Glancy B, Hartnell LM, Combs CA, Femnou A, Sun J, Murphy E, Subramaniam S, Balaban RS (2017) Power grid protection of the muscle mitochondrial reticulum. Cell Rep 19:487–496. https://doi.org/10.1016/j.celrep.2017.03.063
Tezze C, Romanello V, Desbats MA, Fadini GP, Albiero M, Favaro G, Ciciliot S, Soriano ME, Morbidoni V, Cerqua C et al (2017) Age-associated loss of OPA1 in muscle impacts muscle mass, metabolic homeostasis, systemic inflammation, and epithelial senescence. Cell Metab 25:1374–138.e1376. https://doi.org/10.1016/j.cmet.2017.04.021
Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G et al (2008) Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27:433–446. https://doi.org/10.1038/sj.emboj.7601963
Romanello V, Sandri M (2021) The connection between the dynamic remodeling of the mitochondrial network and the regulation of muscle mass. Cell Mol Life Sci 78:1305–1328. https://doi.org/10.1007/s00018-020-03662-0
Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC (2003) Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160:189–200. https://doi.org/10.1083/jcb.200211046
Davies VJ, Hollins AJ, Piechota MJ, Yip W, Davies JR, White KE, Nicols PP, Boulton ME, Votruba M (2007) Opa1 deficiency in a mouse model of autosomal dominant optic atrophy impairs mitochondrial morphology, optic nerve structure and visual function. Hum Mol Genet 16:1307–1318. https://doi.org/10.1093/hmg/ddm079
Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC (2010) Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 141:280–289. https://doi.org/10.1016/j.cell.2010.02.026
Bell MB, Bush Z, McGinnis GR, Rowe GC (2019) Adult skeletal muscle deletion of Mitofusin 1 and 2 impedes exercise performance and training capacity. J Appl Physiol 126:341–353. https://doi.org/10.1152/japplphysiol.00719.2018
Rodriguez-Nuevo A, Diaz-Ramos A, Noguera E, Diaz-Saez F, Duran X, Munoz JP, Romero M, Plana N, Sebastian D, Tezze C et al (2018) Mitochondrial DNA and TLR9 drive muscle inflammation upon Opa1 deficiency. Embo J 37. https://doi.org/10.15252/embj.201796553
Sebastian D, Sorianello E, Segales J, Irazoki A, Ruiz-Bonilla V, Sala D, Planet E, Berenguer-Llergo A, Munoz JP, Sanchez-Feutrie M et al (2016) Mfn2 deficiency links age-related sarcopenia and impaired autophagy to activation of an adaptive mitophagy pathway. EMBO J 35:1677–1693. https://doi.org/10.15252/embj.201593084
Crane JD, Devries MC, Safdar A, Hamadeh MJ, Tarnopolsky MA (2010) The effect of aging on human skeletal muscle mitochondrial and intramyocellular lipid ultrastructure. J Gerontol A Biol Sci Med Sci 65:119–128. https://doi.org/10.1093/gerona/glp179
O’Leary MF, Vainshtein A, Iqbal S, Ostojic O, Hood DA (2013) Adaptive plasticity of autophagic proteins to denervation in aging skeletal muscle. Am J Physiol Cell Physiol 304:C422-430. https://doi.org/10.1152/ajpcell.00240.2012
Wyckelsma VL, Levinger I, McKenna MJ, Formosa LE, Ryan MT, Petersen AC, Anderson MJ, Murphy RM (2017) Preservation of skeletal muscle mitochondrial content in older adults: relationship between mitochondria, fibre type and high-intensity exercise training. J Physiol 595:3345–3359. https://doi.org/10.1113/JP273950
Leduc-Gaudet JP, Picard M, St-Jean Pelletier F, Sgarioto N, Auger MJ, Vallee J, Robitaille R, St-Pierre DH, Gouspillou G (2015) Mitochondrial morphology is altered in atrophied skeletal muscle of aged mice. Oncotarget 6:17923–17937. https://doi.org/10.18632/oncotarget.4235
Joseph AM, Adhihetty PJ, Buford TW, Wohlgemuth SE, Lees HA, Nguyen LM, Aranda JM, Sandesara BD, Pahor M, Manini TM et al (2012) The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell 11:801–809. https://doi.org/10.1111/j.1474-9726.2012.00844.x
Tilokani L, Nagashima S, Paupe V, Prudent J (2018) Mitochondrial dynamics: overview of molecular mechanisms. Essays Biochem 62:341–360. https://doi.org/10.1042/EBC20170104
Favaro G, Romanello V, Varanita T, Andrea Desbats M, Morbidoni V, Tezze C, Albiero M, Canato M, Gherardi G, De Stefani D et al (2019) DRP1-mediated mitochondrial shape controls calcium homeostasis and muscle mass. Nat Commun 10:2576. https://doi.org/10.1038/s41467-019-10226-9
Dulac M, Leduc-Gaudet JP, Reynaud O, Ayoub MB, Guerin A, Finkelchtein M, Hussain SN, Gouspillou G (2020) Drp1 knockdown induces severe muscle atrophy and remodelling, mitochondrial dysfunction, autophagy impairment and denervation. J Physiol 598:3691–3710. https://doi.org/10.1113/JP279802
Touvier T, De Palma C, Rigamonti E, Scagliola A, Incerti E, Mazelin L, Thomas JL, D'Antonio M, Politi L, Schaeffer L et al (2015) Muscle-specific Drp1 overexpression impairs skeletal muscle growth via translational attenuation. Cell Death Dis 6:e1663. https://doi.org/10.1038/cddis.2014.595
Leduc-Gaudet JP, Hussain SNA, Barreiro E, Gouspillou G (2021) Mitochondrial dynamics and mitophagy in skeletal muscle health and aging. Int J Mol Sci 22. https://doi.org/10.3390/ijms22158179
Rana A, Oliveira MP, Khamoui AV, Aparicio R, Rera M, Rossiter HB, Walker DW (2017) Promoting Drp1-mediated mitochondrial fission in midlife prolongs healthy lifespan of Drosophila melanogaster. Nat Commun 8:448. https://doi.org/10.1038/s41467-017-00525-4
Lin J, Handschin C, Spiegelman BM (2005) Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 1:361–370. https://doi.org/10.1016/j.cmet.2005.05.004
Handschin C, Spiegelman BM (2008) The role of exercise and PGC1alpha in inflammation and chronic disease. Nature 454:463–469. https://doi.org/10.1038/nature07206
Dillon LM, Rebelo AP, Moraes CT (2012) The role of PGC-1 coactivators in aging skeletal muscle and heart. IUBMB Life 64:231–241. https://doi.org/10.1002/iub.608
Ji LL, Kang C (2015) Role of PGC-1alpha in sarcopenia:etiology and potential intervention - a mini-review. Gerontology 61:139–148. https://doi.org/10.1159/000365947
Kang C, Li Ji L (2012) Role of PGC-1alpha signaling in skeletal muscle health and disease. Ann N Y Acad Sci 1271:110–117. https://doi.org/10.1111/j.1749-6632.2012.06738.x
Kang C, Ji LL (2013) Muscle immobilization and remobilization downregulates PGC-1alpha signaling and the mitochondrial biogenesis pathway. J Appl Physiol (1985) 115:1618–1625. https://doi.org/10.1152/japplphysiol.01354.2012
Kang C, Goodman CA, Hornberger TA, Ji LL (2015) PGC-1alpha overexpression by in vivo transfection attenuates mitochondrial deterioration of skeletal muscle caused by immobilization. FASEB J 29:4092–4106. https://doi.org/10.1096/fj.14-266619
Irimia JM, Guerrero M, Rodriguez-Miguelez P, Cadefau JA, Tesch PA, Cusso R, Fernandez-Gonzalo R (2017) Metabolic adaptations in skeletal muscle after 84 days of bed rest with and without concurrent flywheel resistance exercise. J Appl Physiol (1985) 122:96–103. https://doi.org/10.1152/japplphysiol.00521.2016
Brocca L, Cannavino J, Coletto L, Biolo G, Sandri M, Bottinelli R, Pellegrino MA (2012) The time course of the adaptations of human muscle proteome to bed rest and the underlying mechanisms. J Physiol 590:5211–5230. https://doi.org/10.1113/jphysiol.2012.240267
Leick L, Lyngby SS, Wojtaszewski JF, Pilegaard H (2010) PGC-1alpha is required for training-induced prevention of age-associated decline in mitochondrial enzymes in mouse skeletal muscle. Exp Gerontol 45:336–342. https://doi.org/10.1016/j.exger.2010.01.011
Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN et al (2002) Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418:797–801. https://doi.org/10.1038/nature00904
Mortensen OH, Frandsen L, Schjerling P, Nishimura E, Grunnet N (2006) PGC-1alpha and PGC-1beta have both similar and distinct effects on myofiber switching toward an oxidative phenotype. Am J Physiol Endocrinol Metab 291:E807-816. https://doi.org/10.1152/ajpendo.00591.2005
Tadaishi M, Miura S, Kai Y, Kano Y, Oishi Y, Ezaki O (2011) Skeletal muscle-specific expression of PGC-1alpha-b, an exercise-responsive isoform, increases exercise capacity and peak oxygen uptake. PLoS One 6:e28290. https://doi.org/10.1371/journal.pone.0028290
Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C et al (2005) PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 3:e101. https://doi.org/10.1371/journal.pbio.0030101
Zechner C, Lai L, Zechner JF, Geng T, Yan Z, Rumsey JW, Collia D, Chen Z, Wozniak DF, Leone TC et al (2010) Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell Metab 12:633–642. https://doi.org/10.1016/j.cmet.2010.11.008
Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM (2006) PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA 103:16260–16265. https://doi.org/10.1073/pnas.0607795103
Wang J, Wang F, Zhang P, Liu H, He J, Zhang C, Fan M, Chen X (2017) PGC-1alpha over-expression suppresses the skeletal muscle atrophy and myofiber-type composition during hindlimb unloading. Biosci Biotechnol Biochem 81:500–513. https://doi.org/10.1080/09168451.2016.1254531
Cannavino J, Brocca L, Sandri M, Grassi B, Bottinelli R, Pellegrino MA (2015) The role of alterations in mitochondrial dynamics and PGC-1alpha over-expression in fast muscle atrophy following hindlimb unloading. J Physiol 593:1981–1995. https://doi.org/10.1113/jphysiol.2014.286740
Wenz T (2011) Mitochondria and PGC-1alpha in aging and age-associated diseases. J Aging Res 2011:810619. https://doi.org/10.4061/2011/810619
Yang S, Loro E, Wada S, Kim B, Tseng WJ, Li K, Khurana TS, Arany Z (2020) Functional effects of muscle PGC-1alpha in aged animals. Skelet Muscle 10:14. https://doi.org/10.1186/s13395-020-00231-8
Ling C, Poulsen P, Carlsson E, Ridderstrale M, Almgren P, Wojtaszewski J, Beck-Nielsen H, Groop L, Vaag A (2004) Multiple environmental and genetic factors influence skeletal muscle PGC-1alpha and PGC-1beta gene expression in twins. J Clin Invest 114:1518–1526. https://doi.org/10.1172/JCI21889
Garcia S, Nissanka N, Mareco EA, Rossi S, Peralta S, Diaz F, Rotundo RL, Carvalho RF, Moraes CT (2018) Overexpression of PGC-1alpha in aging muscle enhances a subset of young-like molecular patterns. Aging Cell 17. https://doi.org/10.1111/acel.12707
Glick D, Barth S, Macleod KF (2010) Autophagy: cellular and molecular mechanisms. J Pathol 221:3–12. https://doi.org/10.1002/path.2697
Sandri M (2010) Autophagy in skeletal muscle. FEBS Lett 584:1411–1416. https://doi.org/10.1016/j.febslet.2010.01.056
Gouspillou G, Godin R, Piquereau J, Picard M, Mofarrahi M, Mathew J, Purves-Smith FM, Sgarioto N, Hepple RT, Burelle Y et al (2018) Protective role of Parkin in skeletal muscle contractile and mitochondrial function. J Physiol 596:2565–2579. https://doi.org/10.1113/JP275604
Peker N, Donipadi V, Sharma M, McFarlane C, Kambadur R (2018) Loss of Parkin impairs mitochondrial function and leads to muscle atrophy. Am J Physiol Cell Physiol 315:C164–C185. https://doi.org/10.1152/ajpcell.00064.2017
Drummond MJ, Addison O, Brunker L, Hopkins PN, McClain DA, LaStayo PC, Marcus RL (2014) Downregulation of E3 ubiquitin ligases and mitophagy-related genes in skeletal muscle of physically inactive, frail older women: a cross-sectional comparison. J Gerontol A Biol Sci Med Sci 69:1040–1048. https://doi.org/10.1093/gerona/glu004
Leduc-Gaudet JP, Reynaud O, Hussain SN, Gouspillou G (2019) Parkin overexpression protects from ageing-related loss of muscle mass and strength. J Physiol 597:1975–1991. https://doi.org/10.1113/JP277157
Rana A, Rera M, Walker DW (2013) Parkin overexpression during aging reduces proteotoxicity, alters mitochondrial dynamics, and extends lifespan. Proc Natl Acad Sci USA 110:8638–8643. https://doi.org/10.1073/pnas.1216197110
Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M (2013) Mechanisms regulating skeletal muscle growth and atrophy. FEBS J 280:4294–4314. https://doi.org/10.1111/febs.12253
Jackman RW, Kandarian SC (2004) The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol 287:C834-843. https://doi.org/10.1152/ajpcell.00579.2003
Fry CS, Rasmussen BB (2011) Skeletal muscle protein balance and metabolism in the elderly. Curr Aging Sci 4:260–268. https://doi.org/10.2174/1874609811104030260
Burd NA, Gorissen SH, van Loon LJ (2013) Anabolic resistance of muscle protein synthesis with aging. Exerc Sport Sci Rev 41:169–173. https://doi.org/10.1097/JES.0b013e318292f3d5
Schiaffino S, Mammucari C (2011) Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet Muscle 1(1):4. https://doi.org/10.1186/2044-5040-1-4
Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL (2004) Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117(3):399–412. https://doi.org/10.1016/s0092-8674(04)00400-3
Egerman MA, Glass DJ (2014) Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol 49:59–68. https://doi.org/10.3109/10409238.2013.857291
Glass DJ (2003) Molecular mechanisms modulating muscle mass. Trends Mol Med 9:344–350
Wen Y, Alimov AP, McCarthy JJ (2016) Ribosome biogenesis is necessary for skeletal muscle hypertrophy. Exerc Sport Sci Rev 44:110–115. https://doi.org/10.1249/JES.0000000000000082
Yoon MS (2017) mTOR as a key regulator in maintaining skeletal muscle mass. Front Physiol 8:788. https://doi.org/10.3389/fphys.2017.00788
Hornberger T, Chu W, Mak Y, Hsiung J, Huang S, Chien S (2006) The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle. Proc Natl Acad Sci 103:4741–4746
Frost RA, Lang CH (2012) Multifaceted role of insulin-like growth factors and mammalian target of rapamycin in skeletal muscle. Endocrinol Metab Clin North Am 41(297–322):vi. https://doi.org/10.1016/j.ecl.2012.04.012
Brook MS, Wilkinson DJ, Mitchell WK, Lund JN, Phillips BE, Szewczyk NJ, Greenhaff PL, Smith K, Atherton PJ (2016) Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans. J Physiol 594:7399–7417. https://doi.org/10.1113/JP272857
Prod’homme M, Balage M, Debras E, Farges MC, Kimball S, Jefferson L, Grizard J (2005) Differential effects of insulin and dietary amino acids on muscle protein synthesis in adult and old rats. J Physiol 563:235–248. https://doi.org/10.1113/jphysiol.2004.068841
Shad BJ, Thompson JL, Breen L (2016) Does the muscle protein synthetic response to exercise and amino acid-based nutrition diminish with advancing age? A systematic review. Am J Physiol Endocrinol Metab 311:E803–E817. https://doi.org/10.1152/ajpendo.00213.2016
Holloway GP, Holwerda AM, Miotto PM, Dirks ML, Verdijk LB, van Loon LJC (2018) Age-associated impairments in mitochondrial ADP sensitivity contribute to redox stress in senescent human skeletal muscle. Cell Rep 22:2837–2848. https://doi.org/10.1016/j.celrep.2018.02.069
Clavel S, Siffroi-Fernandez S, Coldefy AS, Boulukos K, Pisani DF, Derijard B (2010) Regulation of the intracellular localization of Foxo3a by stress-activated protein kinase signaling pathways in skeletal muscle cells. Mol Cell Biol 30:470–480. https://doi.org/10.1128/MCB.00666-09
Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ (2004) The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14:395–403
Marzetti E, Leeuwenburgh C (2006) Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol 41:1234–1238. https://doi.org/10.1016/j.exger.2006.08.011
Primeau AJ, Adhihetty PJ, Hood DA (2002) Apoptosis in heart and skeletal muscle. Can J Appl Physiol 27:349–395. https://doi.org/10.1139/h02-020
Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M et al (2005) Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122:221–233. https://doi.org/10.1016/j.cell.2005.05.011
Khaper N, Singal PK (2001) Modulation of oxidative stress by a selective inhibition of angiotensin II type 1 receptors in MI rats. J Am Coll Cardiol 37:1461–1466. https://doi.org/10.1016/S0735-1097(01)01126-3
Sia YT, Parker TG, Liu P, Tsoporis JN, Adam A, Rouleau JL (2002) Improved post-myocardial infarction survival with probucol in rats:effects on left ventricular function, morphology, cardiac oxidative stress and cytokine expression. J Am Coll Cardiol 39:148–156. https://doi.org/10.1016/S0735-1097(01)01709-0
Baker DJ, Hepple RT (2006) Elevated caspase and AIF gene expression correlate with progression of sarcopenia during aging in male F344BN rats. Exp Gerontol 41:1149–1156. https://doi.org/10.1016/j.exger.2006.08.007
Tamilselvan J, Jayaraman G, Sivarajan K, Panneerselvam C (2007) Age-dependent upregulation of p53 and cytochrome c release and susceptibility to apoptosis in skeletal muscle fiber of aged rats: role of carnitine and lipoic acid. Free Radic Biol Med 43:1656–1669. https://doi.org/10.1016/j.freeradbiomed.2007.08.028
Phillips T, Leeuwenburgh C (2005) Muscle fiber-specific apoptosis and TNF-α signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J 19:1–33. https://doi.org/10.1096/fj.04-2870fje
Snijders T, Verdijk LB, van Loon LJ (2009) The impact of sarcopenia and exercise training on skeletal muscle satellite cells. Ageing Res Rev 8:328–338. https://doi.org/10.1016/j.arr.2009.05.003
Yoshimura Y, Wakabayashi H, Yamada M, Kim H, Harada A, Arai H (2017) Interventions for treating sarcopenia: a systematic review and meta-analysis of randomized controlled studies. J Am Med Dir Assoc 18(553):e551–553.e516. https://doi.org/10.1016/j.jamda.2017.03.019
Phu S, Boersma D, Duque G (2015) Exercise and sarcopenia. J Clin Densitom 18:488–492
Cannataro R, Cione E, Bonilla DA, Cerullo G, Angelini F, D'Antona G (2022) Strength training in elderly: an useful tool against sarcopenia. Front Sports Act Living 4:950949. https://doi.org/10.3389/fspor.2022.950949
Kemmler W, Weineck M, Kohl M, von Stengel S, Giessing J, Frohlich M, Schoene D (2020) High intensity resistance exercise training to improve body composition and strength in older men with osteosarcopenia. Results of the randomized controlled Franconian osteopenia and sarcopenia trial (FrOST). Front Sports Act Living 2:4. https://doi.org/10.3389/fspor.2020.00004
Morton RW, Traylor DA, Weijs PJM, Phillips SM (2018) Defining anabolic resistance: implications for delivery of clinical care nutrition. Curr Opin Crit Care 24:124–130. https://doi.org/10.1097/MCC.0000000000000488
Pennings B, Groen B, de Lange A, Gijsen AP, Zorenc AH, Senden JM, van Loon LJ (2012) Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol Metab 302:E992-999. https://doi.org/10.1152/ajpendo.00517.2011
Fritzen AM, Thogersen FD, Qadri KAN, Krag T, Sveen ML, Vissing J, Jeppesen TD (2020) Preserved capacity for adaptations in strength and muscle regulatory factors in elderly in response to resistance exercise training and deconditioning. J Clin Med 9. https://doi.org/10.3390/jcm9072188
Domingues-Faria C, Vasson M-P, Goncalves-Mendes N, Boirie Y, Walrand S (2016) Skeletal muscle regeneration and impact of aging and nutrition. Ageing Res Rev 26:22–36
Lo JH, U KP, Yiu T, Ong MT, Lee WY, (2020) Sarcopenia: current treatments and new regenerative therapeutic approaches. J Orthop Translat 23:38–52. https://doi.org/10.1016/j.jot.2020.04.002
McLeod JC, Stokes T, Phillips SM (2019) Resistance exercise training as a primary countermeasure to age-related chronic disease. Front Physiol 10:645. https://doi.org/10.3389/fphys.2019.00645
Rivera-Torres S, Fahey TD, Rivera MA (2019) Adherence to exercise programs in older adults: informative report. Gerontol Geriatr Med 5:2333721418823604. https://doi.org/10.1177/2333721418823604
Morley JE (2017) Hormones and sarcopenia. Curr Pharm Des 23:4484–4492. https://doi.org/10.2174/1381612823666161123150032
Camporez JP, Petersen MC, Abudukadier A, Moreira GV, Jurczak MJ, Friedman G, Haqq CM, Petersen KF, Shulman GI (2016) Anti-myostatin antibody increases muscle mass and strength and improves insulin sensitivity in old mice. Proc Natl Acad Sci USA 113:2212–2217. https://doi.org/10.1073/pnas.1525795113
Ascenzi F, Barberi L, Dobrowolny G, Villa Nova Bacurau A, Nicoletti C, Rizzuto E, Rosenthal N, Scicchitano BM, Musaro A (2019) Effects of IGF-1 isoforms on muscle growth and sarcopenia. Aging Cell 18:e12954. https://doi.org/10.1111/acel.12954
Papanicolaou DA, Ather SN, Zhu H, Zhou Y, Lutkiewicz J, Scott BB, Chandler J (2013) A phase IIA randomized, placebo-controlled clinical trial to study the efficacy and safety of the selective androgen receptor modulator (SARM), MK-0773 in female participants with sarcopenia. J Nutr Health Aging 17:533–543. https://doi.org/10.1007/s12603-013-0335-x
Christiansen AR, Lipshultz LI, Hotaling JM, Pastuszak AW (2020) Selective androgen receptor modulators: the future of androgen therapy? Transl Androl Urol 9:S135–S148. https://doi.org/10.21037/tau.2019.11.02
Cesari M, Fielding R, Benichou O, Bernabei R, Bhasin S, Guralnik JM, Jette A, Landi F, Pahor M, Rodriguez-Manas L et al (2015) Pharmacological interventions in frailty and sarcopenia: report by the International Conference on Frailty and Sarcopenia Research Task Force. J Frailty Aging 4:14–120. https://doi.org/10.14283/jfa.2015.64
Correia-Melo C, Marques FD, Anderson R, Hewitt G, Hewitt R, Cole J, Carroll BM, Miwa S, Birch J, Merz A et al (2016) Mitochondria are required for pro-ageing features of the senescent phenotype. Embo j 35:724–742. https://doi.org/10.15252/embj.201592862
Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J (2003) Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol 5:741–747. https://doi.org/10.1038/ncb1024
Kubat GB, Ulger O, Akin S (2021) Requirements for successful mitochondrial transplantation. J Biochem Mol Toxicol 35:e22898. https://doi.org/10.1002/jbt.22898
McCully JD, Cowan DB, Emani SM, Del Nido PJ (2017) Mitochondrial transplantation: from animal models to clinical use in humans. Mitochondrion 34:127–134. https://doi.org/10.1016/j.mito.2017.03.004
Ali Pour P, Kenney MC, Kheradvar A (2020) Bioenergetics consequences of mitochondrial transplantation in cardiomyocytes. Journal of the American Heart Association 9:e014501. https://doi.org/10.1161/jaha.119.014501
Amador-Martínez I, Hernández-Cruz EY, Jiménez-Uribe AP, Sánchez-Lozada LG, Aparicio-Trejo OE, Tapia E, Barrera-Chimal J, Pedraza-Chaverri J (2021) Mitochondrial transplantation: is it a feasible therapy to prevent the cardiorenal side effects of cisplatin? Future Pharmacology 1:3–26
Clark MA, Shay JW (1982) Mitochondrial transformation of mammalian cells. Nature 295:605–607. https://doi.org/10.1038/295605a0
Hsu CH, Roan JN, Fang SY, Chiu MH, Cheng TT, Huang CC, Lin MW, Lam CF (2022) Transplantation of viable mitochondria improves right ventricular performance and pulmonary artery remodeling in rats with pulmonary arterial hypertension. J Thorac Cardiovasc Surg 163:e361–e373. https://doi.org/10.1016/j.jtcvs.2020.08.014
Shin B, Saeed MY, Esch JJ, Guariento A, Blitzer D, Moskowitzova K, Ramirez-Barbieri G, Orfany A, Thedsanamoorthy JK, Cowan DB et al (2019) A novel biological strategy for myocardial protection by intracoronary delivery of mitochondria: safety and efficacy. JACC Basic to translational science 4:871–888. https://doi.org/10.1016/j.jacbts.2019.08.007
Mohammadalipour A, Dumbali SP, Wenzel PL (2020) Mitochondrial transfer and regulators of mesenchymal stromal cell function and therapeutic efficacy. Front Cell Dev Biol 8. https://doi.org/10.3389/fcell.2020.603292
Torralba D, Baixauli F, Sánchez-Madrid F (2016) Mitochondria know no boundaries: mechanisms and functions of intercellular mitochondrial transfer. Front Cell Dev Biol 4. https://doi.org/10.3389/fcell.2016.00107
Austefjord MW, Gerdes HH, Wang X (2014) Tunneling nanotubes: diversity in morphology and structure. Commun Integr Biol 7:e27934. https://doi.org/10.4161/cib.27934
Gurke S, Barroso JF, Gerdes HH (2008) The art of cellular communication: tunneling nanotubes bridge the divide. Histochem Cell Biol 129:539–550. https://doi.org/10.1007/s00418-008-0412-0
Jackson MV, Morrison TJ, Doherty DF, McAuley DF, Matthay MA, Kissenpfennig A, O’Kane CM, Krasnodembskaya AD (2016) Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem cells (Dayton, Ohio) 34:2210–2223. https://doi.org/10.1002/stem.2372
Koyanagi M, Brandes RP, Haendeler J, Zeiher AM, Dimmeler S (2005) Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: a novel mechanism for cell fate changes? Circ Res 96:1039–1041. https://doi.org/10.1161/01.RES.0000168650.23479.0c
Pilling AD, Horiuchi D, Lively CM, Saxton WM (2006) Kinesin-1 and dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol Biol Cell 17:2057–2068. https://doi.org/10.1091/mbc.e05-06-0526
Spees JL, Olson SD, Whitney MJ, Prockop DJ (2006) Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci USA 103:1283–1288. https://doi.org/10.1073/pnas.0510511103
Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, Kumar M, Rehman R, Tiwari BK, Jha KA, Barhanpurkar AP et al (2014) Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. Embo j 33:994–1010. https://doi.org/10.1002/embj.201386030
Liu Z, Sun Y, Qi Z, Cao L, Ding S (2022) Mitochondrial transfer/transplantation: an emerging therapeutic approach for multiple diseases. Cell Biosci 12:66. https://doi.org/10.1186/s13578-022-00805-7
Shanmughapriya S, Langford D, Natarajaseenivasan K (2020) Inter and intracellular mitochondrial trafficking in health and disease. Ageing Res Rev 62:101128. https://doi.org/10.1016/j.arr.2020.101128
Ribeiro-Rodrigues TM, Martins-Marques T, Morel S, Kwak BR, Girão H (2017) Role of connexin 43 in different forms of intercellular communication - gap junctions, extracellular vesicles and tunnelling nanotubes. J Cell Sci 130:3619–3630. https://doi.org/10.1242/jcs.200667
Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J (2012) Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med 18:759–765. https://doi.org/10.1038/nm.2736
Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, Stolz DB, Watkins SC, Di YP, Leikauf GD et al (2015) Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun 6:8472. https://doi.org/10.1038/ncomms9472
Valenti D, Vacca RA, Moro L, Atlante A (2021) Mitochondria can cross cell boundaries: an overview of the biological relevance, pathophysiological implications and therapeutic perspectives of intercellular mitochondrial transfer. Int J Mol Sci 22:8312
Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH (2016) Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535:551–555. https://doi.org/10.1038/nature18928
Emani SM, Piekarski BL, Harrild D, del Nido PJ, McCully JD (2017) Autologous mitochondrial transplantation for dysfunction after ischemia-reperfusion injury. J Thorac Cardiovasc Surg 154:286–289. https://doi.org/10.1016/j.jtcvs.2017.02.018
Guariento A, Piekarski BL, Doulamis IP, Blitzer D, Ferraro AM, Harrild DM, Zurakowski D, del Nido PJ, McCully JD, Emani SM (2021) Autologous mitochondrial transplantation for cardiogenic shock in pediatric patients following ischemia-reperfusion injury. J Thorac Cardiovasc Surg 162:992–1001. https://doi.org/10.1016/j.jtcvs.2020.10.151
Zhang Z, Ma Z, Yan C, Pu K, Wu M, Bai J, Li Y, Wang Q (2019) Muscle-derived autologous mitochondrial transplantation: a novel strategy for treating cerebral ischemic injury. Behav Brain Res 356:322–331. https://doi.org/10.1016/j.bbr.2018.09.005
Huang PJ, Kuo CC, Lee HC, Shen CI, Cheng FC, Wu SF, Chang JC, Pan HC, Lin SZ, Liu CS et al (2016) Transferring xenogenic mitochondria provides neural protection against ischemic stress in ischemic rat brains. Cell Transplant 25:913–927. https://doi.org/10.3727/096368915x689785
Guariento A, Blitzer D, Doulamis I, Shin B, Moskowitzova K, Orfany A, Ramirez-Barbieri G, Staffa SJ, Zurakowski D, Del Nido PJ et al (2020) Preischemic autologous mitochondrial transplantation by intracoronary injection for myocardial protection. J Thorac Cardiovasc Surg 160:e15–e29. https://doi.org/10.1016/j.jtcvs.2019.06.111
Moskowitzova K, Shin B, Liu K, Ramirez-Barbieri G, Guariento A, Blitzer D, Thedsanamoorthy JK, Yao R, Snay ER, Inkster JAH et al (2019) Mitochondrial transplantation prolongs cold ischemia time in murine heart transplantation. J Heart Lung Transplant 38:92–99. https://doi.org/10.1016/j.healun.2018.09.025
Lin HC, Liu SY, Lai HS, Lai IR (2013) Isolated mitochondria infusion mitigates ischemia-reperfusion injury of the liver in rats. Shock 39:304–310. https://doi.org/10.1097/SHK.0b013e318283035f
Lee AR, Woo JS, Lee SY, Na HS, Cho KH, Lee YS, Lee JS, Kim SA, Park SH, Kim SJ et al (2022) Mitochondrial transplantation ameliorates the development and progression of osteoarthritis. Immune Net 22:e14. https://doi.org/10.4110/in.2022.22.e14
Javani G, Babri S, Farajdokht F, Ghaffari-Nasab A, Mohaddes G (2022) Mitochondrial transplantation improves anxiety- and depression-like behaviors in aged stress-exposed rats. Mech Ageing Dev 202:111632. https://doi.org/10.1016/j.mad.2022.111632
Zhang Z, Wei D, Li Z, Guo H, Wu Y, Feng J (2022) Hippocampal mitochondrial transplantation alleviates age-associated cognitive decline via enhancing Wnt signaling and neurogenesis. Comput Intell Neurosci 2022:9325302. https://doi.org/10.1155/2022/9325302
Wu HC, Fan X, Hu CH, Chao YC, Liu CS, Chang JC, Sen Y (2020) Comparison of mitochondrial transplantation by using a stamp-type multineedle injector and platelet-rich plasma therapy for hair aging in naturally aging mice. Biomed Pharmacother 130:110520. https://doi.org/10.1016/j.biopha.2020.110520
Diaz-Vegas A, Sanchez-Aguilera P, Krycer JR, Morales PE, Monsalves-Alvarez M, Cifuentes M, Rothermel BA, Lavandero S (2020) Is mitochondrial dysfunction a common root of noncommunicable chronic diseases? Endocrine Rev 41. https://doi.org/10.1210/endrev/bnaa005
Author information
Authors and Affiliations
Contributions
Ibrahim Turkel, Berkay Ozerklig, and Gokhan Burcin Kubat contributed to the conception and design of the review. Ibrahim Turkel, Berkay Ozerklig, Merve Yılmaz, Oner Ulger, and Gokhan Burcin Kubat performed the literature review and wrote the draft of the manuscript. Ibrahim Turkel, Berkay Ozerklig, and Gokhan Burcin Kubat designed the figures and table. Meltem Tuncer revised the draft of the manuscript. All authors critically revised the manuscript and approved the final version of the submitted manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Turkel, I., Ozerklig, B., Yılmaz, M. et al. Mitochondrial transplantation as a possible therapeutic option for sarcopenia. J Mol Med 101, 645–669 (2023). https://doi.org/10.1007/s00109-023-02326-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-023-02326-3