Abstract
Background
The osteocyte-derived sclerostin has been shown to play a key inhibitor role in determining the normal extent of bone formation, and it consequently protects against the deleterious effects of uncontrolled bone growth. Sclerostin has been demonstrated to be upregulated during vascular smooth muscle cell calcification in vitro and has recently been identified in the human aorta at the protein level. Whether the effects of sclerostin on bone turnover and its vascular expression also translate into clinically significant changes in arteriovenous fistula patency is unknown.
Patients and methods
The primary outcome was loss of unassisted arteriovenous fistula patency, defined as arteriovenous fistula thrombosis or need for intervention. In this prospective cohort study, 350 prevalent hemodialysis patients were followed up for 12 months. Serum sclerostin levels were measured and arteriovenous fistula calcification was detected using a 64-detector computerized tomographic scanner.
Results
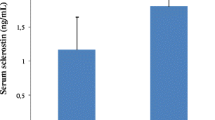
Patients with calcified arteriovenous fistula had higher serum sclerostin levels than patients without. Overall, 26 % of the patients reached the outcome during the follow-up. The 12-month arteriovenous fistula survival was reduced in patients with calcified arteriovenous fistulas. Patients with serum sclerostin levels above median levels at the start of the observation period had a worse arteriovenous fistula survival. Multivariable-adjusted Cox regression analyses revealed that only presence of arteriovenous fistula calcification and serum C-reactive protein level independently predicted loss of unassisted arteriovenous fistula patency.
Conclusion
Our study suggests that the detection of arteriovenous fistula calcification and serum C-reactive protein levels might be useful for identifying patients at an increased risk for loss of unassisted arteriovenous fistula patency.
Zusammenfassung
Hintergrund
Das in Osteozyten gebildete Sclerostin spielt nachgewiesenermaßen als Inhibitor eine Schlüsselrolle dabei, das normale Ausmaß der Knochenbildung festzulegen, und dient somit als Schutz vor den schädlichen Wirkungen eines unkontrollierten Knochenwachstums. Sclerostin wird, wie gezeigt wurde, während der Kalzifizierung von vaskulären glatten Muskelzellen in vitro hochreguliert und ist auf Proteinebene vor Kurzem in der menschlichen Aorta nachgewiesen worden. Ob die Wirkung von Sclerostin auf den Knochenstoffwechsel und seine Expression in Gefäßen auch von klinischer Bedeutung für Veränderungen bei der Durchgängigkeit eines arteriovenösen Shunts ist, ist nicht bekannt.
Methoden
Der primäre Endpunkt war der Verlust der Durchgängigkeit des arteriovenösen Shunts ohne weitere Maßnahmen, was als Shuntthrombose oder Notwendigkeit der Intervention definiert war. In dieser prospektiven Kohortenstudie wurden 350 Patienten mit bestehender Hämodialysetherapie 12 Monate lang nachbeobachtet. Es wurden die Sclerostinwerte im Serum bestimmt und die Kalzifizierung des arteriovenösen Shunts mit einem 64-Zeilen-Computertomographen ermittelt.
Ergebnisse
Bei Patienten mit Kalzifizierung des arteriovenösen Shunts war der Sclerostinwert im Serum höher als bei Patienten ohne Shuntkalzifizierung. Insgesamt trat der Endpunkt bei 26 % der Patienten während des Nachbeobachtungszeitraums ein. Das 12-Monats-Überleben des arteriovenösen Shunts war bei Patienten mit Shuntkalzifizierung vermindert. Bei Patienten mit Sclerostinwerten im Serum oberhalb des Durchschnittswerts zu Beginn der Beobachtungsphase war das Überleben des arteriovenösen Shunts schlechter. Die unter Berücksichtigung mehrerer Variablen durchgeführte Cox-Regressionsanalyse ergab, dass nur das Vorliegen einer Shuntkalzifizierung und der Wert für C-reaktives Protein (CRP) im Serum den Verlust der Shuntdurchgängigkeit ohne weitere Maßnahmen unabhängig vorhersagten.
Schlussfolgerungen
Der vorliegenden Studie zufolge können die Diagnosestellung einer Kalzifizierung des arteriovenösen Shunts und die Bestimmung des CRP im Serum bei der Erkennung von Patienten mit erhöhtem Risiko für den Verlust der Durchgängigkeit des Shunts ohne weitere Maßnahmen von Nutzen sein.




Similar content being viewed by others
References
Feldman HI, Kobrin S, Wasserstein A (1996) Hemodialysis vascular access morbidity. J Am Soc Nephrol 7:523–535
Polkinghorne KR, McDonald SP, Atkins RC et al (2004) Vascular access and all-cause mortality: a propensity score analysis. J Am Soc Nephrol 15:477–486
Roy-Chaudhury P, Sukhatme VP, Cheung AK (2006) Hemodialysis vascular access dysfunction: a cellular and molecular viewpoint. J Am Soc Nephrol 17:1112–1127
Krishnan V, Bryant HU, Macdougald OA (2006) Regulation of bone mass by Wnt signaling. J Clin Invest 116:1202–1209
Drüeke TB, Lafage-Proust MH (2011) Sclerostin: just one more player in renal bone disease? Clin J Am Soc Nephrol 6:700–703
Cejka D, Herberth J, Branscum AJ et al (2011) Sclerostin and Dickkopf-1 in renal osteodystrophy. Clin J Am Soc Nephrol 6:877–882
Zhu D, Mackenzie NC, Millán JL et al (2011) The appearance and modulation of osteocyte markerexpression during calcification of vascular smooth muscle cells. PLoS One 6(5):e19595
Tessitore N, Bedogna V, Gammaro L et al (2003) Diagnostic accuracy of ultrasound dilution access blood flow measurement in detecting stenosisand predicting thrombosis in native forearm fistulas for hemodialysis. Am J Kidney Dis 42:331–341
Cejka D, Jäger-Lansky A, Kieweg H et al (2012) Sclerostin serum levels correlate positively with bone mineral density and microarchitecture in haemodialysis patients. Nephrol Dial Transplant 27:226–230
Agatston AS, Janowitz WR, Hildner FJ et al (1990) Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15:827–832
Adragao T, Pires A, Lucas C et al (2004) A simple vascular calcification score predicts cardiovascular risk in haemodialysis patients. Nephrol Dial Transplant 19:1480–1488
Schlieper G, Krüger T, Djuric Z et al (2008) Vascular access calcification predicts mortality in hemodialysis patients. Kidney Int 74(12):1582–1587
Johnson RC, Leopold JA, Loscalzo J (2006) Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res 99(10):1044–1059
Lok CE, Allon M, Moist L et al (2006) Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I). J Am Soc Nephrol 17:3204–3212
Lin CJ, Pan CF, Liu HL et al (2012) The role of protein-bound uremic toxins on peripheral artery disease and vascular access failure in patients on hemodialysis. Atherosclerosis 225(1):173–179
Olsson LF, Odselius R, Ribbe E et al (2001) Evidence of calcium phosphate depositions in stenotic arteriovenous fistulas. Am J Kidney Dis 38(2):377–383
Becker A, Epple M, Müller KM et al (2004) A comparative study of clinically well-characterized human atherosclerotic plaques with histological, chemical, and ultrastructural methods. J Inorg Biochem 98(12):2032–2038
Poole KE, Bezooijen RL van, Loveridge N et al (2005) Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J 19:1842–1844
Shao JS, Cheng SL, Pingsterhaus JM et al (2005) Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest 115:1210–1220
Claes KJ, Viaene L, Heye S et al (2013) Sclerostin: Another vascular calcification inhibitor? J Clin Endocrinol Metab (Published online before print June 20, 2013, doi:10.1210/jc.2013-1521)
Thambiah S, Roplekar R, Manghat P et al (2012) Circulating sclerostin and Dickkopf-1 (DKK1) in predialysis chronic kidney disease (CKD): relationship with bone density and arterial stiffness. Calcif Tissue Int 90(6):473–480
Drake MT, Srinivasan B, Mödder UI et al (2010) Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab 95:5056–5062
Viaene L, Behets GJ, Claes K et al (2013) Sclerostin: another bone-related protein related to all-cause mortality in haemodialysis? Nephrol Dial Transplant (Epub ahead of print)
Roca-Tey R, Páez R, Rivas A et al (2009) Prevalence and functional effect of arteriovenous fistula calcifications, evaluated by spiral CT in chronic haemodialysis patients. Nefrologia 29(3):214–221
Wali MA, Eid RA, Dewan M et al (2006) Pre-existing histopathological changes in the cephalic vein of renal failure patients before arterio-venous fistula (AVF) construction. Ann Thorac Cardiovasc Surg 12(5):341–348
Craig TA, Bhattacharya R, Mukhopadhyay D et al (2010) Sclerostin binds and regulates the activity of cysteine-rich protein 61. Biochem Biophys Res Commun 392:36–40
Motwani JG, Topol EJ (1998) Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation 97(9):916–931
Lee T, Safdar N, Mistry MJ et al (2012) Preexisting venous calcification prior to dialysis vascular access surgery. Semin Dial 25(5):592–595
Bulkley BH, Hutchins GM (1977) Accelerated “atherosclerosis”. A morphologic study of 97 saphenous vein coronary artery bypass grafts. Circulation 55(1):163–169
Campeau L, Enjalbert M, Lespérance J et al (1984) The relation of risk factors to the development of atherosclerosis in saphenous-vein bypass grafts and the progression of disease in the native circulation. A study 10 years after aortocoronary bypass surgery. N Engl J Med 311(21):1329–1332
Kirkpantur A, Arici M, Altun B et al (2008) Association of serum lipid profile and arteriovenous fistula thrombosis in maintenance hemodialysis patients. Blood Purif 26(4):322–332
Liu BC, Li L, Gao M et al (2008) Microinflammation is involved in the dysfunction of arteriovenous fistula in patients with maintenance hemodialysis. Chin Med J (Engl) 121:2157–2161
Stracke S, Konner K, Köstlin I et al (2002) Increased expression of TGF-beta1 and IGF-I in inflammatory stenotic lesions of hemodialysis fistulas. Kidney Int 61:1011–1019
Pasceri V, Willerson JT, Yeh ET (2000) Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation 102:2165–2168
Torzewski J, Torzewski M, Bowyer DE et al (1998) C-reactive protein frequently colocalizes with the terminal complement complex in the intima of early atherosclerotic lesions of human coronary arteries. Arterioscler Thromb Vasc Biol 18:1386–1392
Wang CH, Li SH, Weisel RD et al (2003) C-reactive protein upregulates angiotensin type 1 receptors in vascular smooth muscle. Circulation 107:1783–1790
Mödder UI, Hoey KA, Amin S et al (2011) Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res 26(2):373–379
Compliance with ethical guidelines
Conflict of interest. M. Balcı, A. Kırkpantur, A. Turkvatan, S. Mandıroglu, E. Ozturk, and B. Afsar state that there are no conflicts of interest. All studies on humans described in the present manuscript were carried out with the approval of the responsible ethics committee and in accordance with national law and the Helsinki Declaration of 1975 (in its current, revised form). Informed consent was obtained from all patients included in studies. Consent was obtained from all patients identifiable from images or other information within the manuscript. In the case of underage patients, consent was obtained from a parent or legal guardian.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balcı, M., Kırkpantur, A., Turkvatan, A. et al. Sclerostin as a new key player in arteriovenous fistula calcification. Herz 40, 289–297 (2015). https://doi.org/10.1007/s00059-013-3992-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-013-3992-y