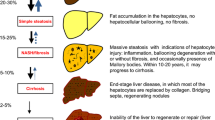
Abstract
Hereditary fructose intolerance (HFI) is a rare inborn disease characterized by a deficiency in aldolase B, which catalyzes the cleavage of fructose 1,6-bisphosphate and fructose 1-phosphate (Fru 1P) to triose molecules. In patients with HFI, ingestion of fructose results in accumulation of Fru 1P and depletion of ATP, which are believed to cause symptoms, such as nausea, vomiting, hypoglycemia, and liver and kidney failure. These sequelae can be prevented by a fructose-restricted diet. Recent studies in aldolase B-deficient mice and HFI patients have provided more insight into the pathogenesis of HFI, in particular the liver phenotype. Both aldolase B-deficient mice (fed a very low fructose diet) and HFI patients (treated with a fructose-restricted diet) displayed greater intrahepatic fat content when compared to controls. The liver phenotype in aldolase B-deficient mice was prevented by reduction in intrahepatic Fru 1P concentrations by crossing these mice with mice deficient for ketohexokinase, the enzyme that catalyzes the synthesis of Fru 1P. These new findings not only provide a potential novel treatment for HFI, but lend insight into the pathogenesis of fructose-induced non-alcoholic fatty liver disease (NAFLD), which has raised to epidemic proportions in Western society. This narrative review summarizes the most recent advances in the pathogenesis of HFI and discusses the implications for the understanding and treatment of fructose-induced NAFLD.


Similar content being viewed by others
References
Chambers RA, Pratt RT (1956) Idiosyncrasy to fructose. Lancet 271(6938):340
Herrs H, Joassin G (1961) Anomalie de l’aldolase hepatique dans l’intolerance au fructose. Enzymol Biol Clin 1:4–14
Li H, Byers HM, Diaz-Kuan A, Vos MB, Hall PL, Tortorelli S, Singh R, Wallenstein MB, Allain M, Dimmock DP, Farrell RM, McCandless S, Gambello MJ (2018) Acute liver failure in neonates with undiagnosed hereditary fructose intolerance due to exposure from widely available infant formulas. Mol Genet Metab 123(4):428–432. https://doi.org/10.1016/j.ymgme.2018.02.016
Sanchez-Lozada LG, Andres-Hernando A, Garcia-Arroyo FE, Cicerchi C, Li N, Kuwabara M, Roncal-Jimenez CA, Johnson RJ, Lanaspa MA (2019) Uric acid activates aldose reductase and the polyol pathway for endogenous fructose and fat production causing development of fatty liver in rats. J Biol Chem. https://doi.org/10.1074/jbc.RA118.006158
Yan LJ (2018) Redox imbalance stress in diabetes mellitus: role of the polyol pathway. Animal Model Exp Med 1(1):7–13. https://doi.org/10.1002/ame2.12001
Odievre M, Gentil C, Gautier M, Alagille D (1978) Hereditary fructose intolerance in childhood. Diagnosis, management, and course in 55 patients. Am J Dis Child 132(6):605–608
Baerlocher K, Gitzelmann R, Steinmann B, Gitzelmann-Cumarasamy N (1978) Hereditary fructose intolerance in early childhood: a major diagnostic challenge. Survey of 20 symptomatic cases. Helv Paediatr Acta 33(6):465–487
Mock DM, Perman JA, Thaler M, Morris RC Jr (1983) Chronic fructose intoxication after infancy in children with hereditary fructose intolerance. A cause of growth retardation. N Engl J Med 309(13):764–770. https://doi.org/10.1056/nejm198309293091305
Ali M, Rellos P, Cox TM (1998) Hereditary fructose intolerance. J Med Genet 35(5):353–365
Lebo RV, Tolan DR, Bruce BD, Cheung MC, Kan YW (1985) Spot-blot analysis of sorted chromosomes assigns a fructose intolerance disease locus to chromosome 9. Cytometry 6(5):478–483. https://doi.org/10.1002/cyto.990060513
Lench NJ, Telford EA, Andersen SE, Moynihan TP, Robinson PA, Markham AF (1996) An EST and STS-based YAC contig map of human chromosome 9q22.3. Genomics 38(2):199–205. https://doi.org/10.1006/geno.1996.0616
Cross NC, de Franchis R, Sebastio G, Dazzo C, Tolan DR, Gregori C, Odievre M, Vidailhet M, Romano V, Mascali G et al (1990) Molecular analysis of aldolase B genes in hereditary fructose intolerance. Lancet 335(8685):306–309
Tolan DR, Brooks CC (1992) Molecular analysis of common aldolase B alleles for hereditary fructose intolerance in North Americans. Biochem Med Metab Biol 48(1):19–25
Cross NC, Stojanov LM, Cox TM (1990) A new aldolase B variant, N334 K, is a common cause of hereditary fructose intolerance in Yugoslavia. Nucleic Acids Res 18(7):1925
Coffee EM, Yerkes L, Ewen EP, Zee T, Tolan DR (2010) Increased prevalence of mutant null alleles that cause hereditary fructose intolerance in the American population. J Inherit Metab Dis 33(1):33–42. https://doi.org/10.1007/s10545-009-9008-7
Dazzo C, Tolan DR (1990) Molecular evidence for compound heterozygosity in hereditary fructose intolerance. Am J Hum Genet 46(6):1194–1199
Cross NC, Tolan DR, Cox TM (1988) Catalytic deficiency of human aldolase B in hereditary fructose intolerance caused by a common missense mutation. Cell 53(6):881–885
James CL, Rellos P, Ali M, Heeley AF, Cox TM (1996) Neonatal screening for hereditary fructose intolerance: frequency of the most common mutant aldolase B allele (A149P) in the British population. J Med Genet 33(10):837–841
Gitzelmann R, Baerlocher K (1973) Vorteile und Nachteile der Fruktose in der Nahrung. Pädiat Fortbildk Praxis 37:40–55
Cox TM (1994) Aldolase B and fructose intolerance. FASEB J 8(1):62–71
Tolan DR (1995) Molecular basis of hereditary fructose intolerance: mutations and polymorphisms in the human aldolase B gene. Hum Mutat 6(3):210–218. https://doi.org/10.1002/humu.1380060303
Hers H (1957) Le métabolisme du fructose. Editions Arscia, Bruxelles
Penhoet EE, Rutter WJ (1971) Catalytic and immunochemical properties of homomeric and heteromeric combinations of aldolase subunits. J Biol Chem 246(2):318–323
Penhoet E, Rajkumar T, Rutter WJ (1966) Multiple forms of fructose diphosphate aldolase in mammalian tissues. Proc Natl Acad Sci USA 56(4):1275–1282
Oppelt SA, Zhang W, Tolan DR (2017) Specific regions of the brain are capable of fructose metabolism. Brain Res 1657:312–322. https://doi.org/10.1016/j.brainres.2016.12.022
Steinmann B, Gitzelmann R (1981) The diagnosis of hereditary fructose intolerance. Helv Paediatr Acta 36(4):297–316
Oberhaensli RD, Rajagopalan B, Taylor DJ, Radda GK, Collins JE, Leonard JV, Schwarz H, Herschkowitz N (1987) Study of hereditary fructose intolerance by use of 31P magnetic resonance spectroscopy. Lancet 2(8565):931–934
Woods HF, Eggleston LV, Krebs HA (1970) The cause of hepatic accumulation of fructose 1-phosphate on fructose loading. Biochem J 119(3):501–510
van den Berghe G, Bronfman M, Vanneste R, Hers HG (1977) The mechanism of adenosine triphosphate depletion in the liver after a load of fructose. A kinetic study of liver adenylate deaminase. Biochem J 162(3):601–609
Kaufmann U, Froesch ER (1973) Inhibition of phosphorylase-a by fructose-1-phosphate, alpha-glycerophosphate and fructose-1,6-diphosphate: explanation for fructose-induced hypoglycaemia in hereditary fructose intolerance and fructose-1,6-diphosphatase deficiency. Eur J Clin Invest 3(5):407–413
Thurston JH, Jones EM, Hauhart RE (1974) Decrease and inhibition of liver glycogen phosphorylase after fructose. An experimental model for the study of hereditary fructose intolerance. Diabetes 23(7):597–604
Van Den Berghe G, Hue L, Hers HG (1973) Effect of administration of the fructose on the glycogenolytic action of glucagon. An investigation of the pathogeny of hereditary fructose intolerance. Biochem J 134(2):637–645
Van den Berghe G (1975) Biochemical aspects of hereditary fructose intolerance. In: Hommes FA, van den Berg CJ (eds) Normal and pathological development of energy metabolism. Academic Press, London, pp 221–228
Dubois RLH, Malaisse-Lagaw F, Toppet M (1965) Etude clinique et anatomo-pathologique de deux cas d’intolerance congenitale au fructose. Pediatrie 20:5–14
Rossier AMG, Colin J, Job J-C, Brault A, Beauvais P, Lemerle J (1966) Intolérance congénitale au fructose. deux cas damiliaux avec étude biochimique in vitro. Arch Fr Pediatr 23:533
Zalitis J, Oliver IT (1967) Inhibition of glucose phosphate isomerase by metabolic intermediates of fructose. Biochem J 102(3):753–759
Froesch E, Prader A, Wolf H, Labhart A (1959) Die hereditare Fructoseintoleranz. Helvetica Paediatrica Acta 14:99–112
Wilkening J, Nowack J, Decker K (1975) The dependence of glucose formation from lactate on the adenosine triphosphate content in the isolated perfused rat liver. Biochim Biophys Acta 392(2):299–309
Gitzelmann R, Steinmann B, Gvd Berghe (1995) Disorders of Fructose Metabolism. In: Scriver C, Beaudet A, Sly W, Valle D (eds) The Metabolic and Molecular Basis of Inherited Disease. McGraw-Hill, New York, pp 905–934
Woods HF, Alberti KG (1972) Dangers of intravenous fructose. Lancet 2(7791):1354–1357
Bergstrom J, Hultman E, Roch-Norlund AE (1968) Lactic acid accumulation in connection with fructose infusion. Acta Med Scand 184(5):359–364
Lanaspa MA, Ishimoto T, Li N, Cicerchi C, Orlicky DJ, Ruzycki P, Rivard C, Inaba S, Roncal-Jimenez CA, Bales ES, Diggle CP, Asipu A, Petrash JM, Kosugi T, Maruyama S, Sanchez-Lozada LG, McManaman JL, Bonthron DT, Sautin YY, Johnson RJ (2013) Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat Commun 4:2434. https://doi.org/10.1038/ncomms3434
Johnson RJ, Rodriguez-Iturbe B, Roncal-Jimenez C, Lanaspa MA, Ishimoto T, Nakagawa T, Correa-Rotter R, Wesseling C, Bankir L, Sanchez-Lozada LG (2014) Hyperosmolarity drives hypertension and CKD–water and salt revisited. Nat Rev Nephrol 10(7):415–420. https://doi.org/10.1038/nrneph.2014.76
Roncal Jimenez CA, Ishimoto T, Lanaspa MA, Rivard CJ, Nakagawa T, Ejaz AA, Cicerchi C, Inaba S, Le M, Miyazaki M, Glaser J, Correa-Rotter R, Gonzalez MA, Aragon A, Wesseling C, Sanchez-Lozada LG, Johnson RJ (2014) Fructokinase activity mediates dehydration-induced renal injury. Kidney Int 86(2):294–302. https://doi.org/10.1038/ki.2013.492
Sward P, Rippe B (2012) Acute and sustained actions of hyperglycaemia on endothelial and glomerular barrier permeability. Acta Physiol (Oxf) 204(3):294–307. https://doi.org/10.1111/j.1748-1716.2011.02343.x
Lyons PA, Gould S, Wise PH, Palmer TN (1991) Activation of erythrocyte aldose reductase in man in response to glycaemic challenge. Diabetes Res Clin Pract 14(1):9–13
Oppelt SA, Sennott EM, Tolan DR (2015) Aldolase-B knockout in mice phenocopies hereditary fructose intolerance in humans. Mol Genet Metab 114(3):445–450. https://doi.org/10.1016/j.ymgme.2015.01.001
Lanaspa MA, Andres-Hernando A, Orlicky DJ, Cicerchi C, Jang C, Li N, Milagres T, Kuwabara M, Wempe MF, Rabinowitz JD, Johnson RJ, Tolan DR (2018) Ketohexokinase C blockade ameliorates fructose-induced metabolic dysfunction in fructose-sensitive mice. J Clin Invest 128(6):2226–2238. https://doi.org/10.1172/JCI94427
Coffee E, Tolan D (2014) Gluconeogenesis. In: Lee B, Scaglia F (eds) Inborn errors of metabolism: from neonatal screening to metabolic pathways. Oxford University Press, New York, p 384
Agius L (2008) Glucokinase and molecular aspects of liver glycogen metabolism. Biochem J 414(1):1–18. https://doi.org/10.1042/BJ20080595
Agius L, Peak M, Van Schaftingen E (1995) The regulatory protein of glucokinase binds to the hepatocyte matrix, but, unlike glucokinase, does not translocate during substrate stimulation. Biochem J 309(Pt 3):711–713
van Schaftingen E, Veiga-da-Cunha M, Niculescu L (1997) The regulatory protein of glucokinase. Biochem Soc Trans 25(1):136–140
van Schaftingen E, Vandercammen A, Detheux M, Davies DR (1992) The regulatory protein of liver glucokinase. Adv Enzyme Regul 32:133–148
Vandercammen A, Van Schaftingen E (1993) Species and tissue distribution of the regulatory protein of glucokinase. Biochem J 294(Pt 2):551–556
Veiga-Da-Cunha M, Detheux M, Watelet N, Van Schaftingen E (1994) Cloning and expression of a Xenopus liver cDNA encoding a fructose-phosphate-insensitive regulatory protein of glucokinase. Eur J Biochem 225(1):43–51
Beck T, Miller BG (2013) Structural basis for regulation of human glucokinase by glucokinase regulatory protein. Biochemistry 52(36):6232–6239. https://doi.org/10.1021/bi400838t
Choi JM, Seo MH, Kyeong HH, Kim E, Kim HS (2013) Molecular basis for the role of glucokinase regulatory protein as the allosteric switch for glucokinase. Proc Natl Acad Sci USA 110(25):10171–10176. https://doi.org/10.1073/pnas.1300457110
Jin ES, Lee MH, Murphy RE, Malloy CR (2018) Pentose phosphate pathway activity parallels lipogenesis but not antioxidant processes in rat liver. Am J Physiol Endocrinol Metab 314(6):E543–E551. https://doi.org/10.1152/ajpendo.00342.2017
Lanaspa MA, Sanchez-Lozada LG, Choi YJ, Cicerchi C, Kanbay M, Roncal-Jimenez CA, Ishimoto T, Li N, Marek G, Duranay M, Schreiner G, Rodriguez-Iturbe B, Nakagawa T, Kang DH, Sautin YY, Johnson RJ (2012) Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J Biol Chem 287(48):40732–40744. https://doi.org/10.1074/jbc.M112.399899
Kim MS, Krawczyk SA, Doridot L, Fowler AJ, Wang JX, Trauger SA, Noh HL, Kang HJ, Meissen JK, Blatnik M, Kim JK, Lai M, Herman MA (2016) ChREBP regulates fructose-induced glucose production independently of insulin signaling. J Clin Invest 126(11):4372–4386. https://doi.org/10.1172/JCI81993
Hannou SA, Haslam DE, McKeown NM, Herman MA (2018) Fructose metabolism and metabolic disease. J Clin Invest 128(2):545–555. https://doi.org/10.1172/JCI96702
Hayward BE, Bonthron DT (1998) Structure and alternative splicing of the ketohexokinase gene. Eur J Biochem 257(1):85–91
Diggle CP, Shires M, Leitch D, Brooke D, Carr IM, Markham AF, Hayward BE, Asipu A, Bonthron DT (2009) Ketohexokinase: expression and localization of the principal fructose-metabolizing enzyme. J Histochem Cytochem 57(8):763–774. https://doi.org/10.1369/jhc.2009.953190
Le MT, Lanaspa MA, Cicerchi CM, Rana J, Scholten JD, Hunter BL, Rivard CJ, Randolph RK, Johnson RJ (2016) Bioactivity-guided identification of botanical inhibitors of ketohexokinase. PLoS One 11(6):e0157458. https://doi.org/10.1371/journal.pone.0157458
Aldamiz-Echevarria L, de Las Heras J, Couce ML, Alcalde C, Vitoria I, Bueno M, Blasco-Alonso J, Concepcion Garcia M, Ruiz M, Suarez R, Andrade F, Villate O (2019) Non-alcoholic fatty liver in hereditary fructose intolerance. Clin Nutr. https://doi.org/10.1016/j.clnu.2019.02.019
Marriott BP, Cole N, Lee E (2009) National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr 139(6):1228S–1235S. https://doi.org/10.3945/jn.108.098277
Simons N, Debray FG, Schaper NC, Kooi ME, Feskens EJM, Hollak CEM, Lindeboom L, Koek GH, Bons JAP, Lefeber DJ, Hodson L, Schalkwijk CG, Stehouwer CDA, Cassiman D, Brouwers M (2019) Patients with aldolase B deficiency are characterized by an increased intrahepatic triglyceride content. J Clin Endocrinol Metab. https://doi.org/10.1210/jc.2018-02795
Adamowicz M, Ploski R, Rokicki D, Morava E, Gizewska M, Mierzewska H, Pollak A, Lefeber DJ, Wevers RA, Pronicka E (2007) Transferrin hypoglycosylation in hereditary fructose intolerance: using the clues and avoiding the pitfalls. J Inherit Metab Dis 30(3):407. https://doi.org/10.1007/s10545-007-0569-z
Quintana E, Sturiale L, Montero R, Andrade F, Fernandez C, Couce ML, Barone R, Aldamiz-Echevarria L, Ribes A, Artuch R, Briones P (2009) Secondary disorders of glycosylation in inborn errors of fructose metabolism. J Inherit Metab Dis 32(Suppl 1):S273–S278. https://doi.org/10.1007/s10545-009-1219-4
Jaeken J, Pirard M, Adamowicz M, Pronicka E, van Schaftingen E (1996) Inhibition of phosphomannose isomerase by fructose 1-phosphate: an explanation for defective N-glycosylation in hereditary fructose intolerance. Pediatr Res 40(5):764–766. https://doi.org/10.1203/00006450-199611000-00017
Foster DW (2012) Malonyl-CoA: the regulator of fatty acid synthesis and oxidation. J Clin Invest 122(6):1958–1959
Brouwers M, Jacobs C, Bast A, Stehouwer CDA, Schaper NC (2015) Modulation of glucokinase regulatory protein: a double-edged sword? Trends Mol Med 21(10):583–594. https://doi.org/10.1016/j.molmed.2015.08.004
Beer NL, Tribble ND, McCulloch LJ, Roos C, Johnson PR, Orho-Melander M, Gloyn AL (2009) The P446L variant in GCKR associated with fasting plasma glucose and triglyceride levels exerts its effect through increased glucokinase activity in liver. Hum Mol Genet 18(21):4081–4088. https://doi.org/10.1093/hmg/ddp357
Mahendran Y, Vangipurapu J, Cederberg H, Stancakova A, Pihlajamaki J, Soininen P, Kangas AJ, Paananen J, Civelek M, Saleem NK, Pajukanta P, Lusis AJ, Bonnycastle LL, Morken MA, Collins FS, Mohlke KL, Boehnke M, Ala-Korpela M, Kuusisto J, Laakso M (2013) Association of ketone body levels with hyperglycemia and type 2 diabetes in 9,398 Finnish men. Diabetes 62(10):3618–3626. https://doi.org/10.2337/db12-1363
Santoro N, Zhang CK, Zhao H, Pakstis AJ, Kim G, Kursawe R, Dykas DJ, Bale AE, Giannini C, Pierpont B, Shaw MM, Groop L, Caprio S (2012) Variant in the glucokinase regulatory protein (GCKR) gene is associated with fatty liver in obese children and adolescents. Hepatology 55(3):781–789. https://doi.org/10.1002/hep.24806
Santoro N, Caprio S, Pierpont B, Name MV, Savoye M, Parks EJ (2015) Hepatic de novo lipogenesis in obese youth is modulated by a common variant in the GCKR gene. J Clin Endocrinol Metab. https://doi.org/10.1210/jc.2015-1587
Speliotes EK, Yerges-Armstrong LM, Wu J, Hernaez R, Kim LJ, Palmer CD, Gudnason V, Eiriksdottir G, Garcia ME, Launer LJ, Nalls MA, Clark JM, Mitchell BD, Shuldiner AR, Butler JL, Tomas M, Hoffmann U, Hwang SJ, Massaro JM, O’Donnell CJ, Sahani DV, Salomaa V, Schadt EE, Schwartz SM, Siscovick DS, Voight BF, Carr JJ, Feitosa MF, Harris TB, Fox CS, Smith AV, Kao WH, Hirschhorn JN, Borecki IB (2011) Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet 7(3):e1001324. https://doi.org/10.1371/journal.pgen.1001324
Orho-Melander M, Melander O, Guiducci C, Perez-Martinez P, Corella D, Roos C, Tewhey R, Rieder MJ, Hall J, Abecasis G, Tai ES, Welch C, Arnett DK, Lyssenko V, Lindholm E, Saxena R, de Bakker PI, Burtt N, Voight BF, Hirschhorn JN, Tucker KL, Hedner T, Tuomi T, Isomaa B, Eriksson KF, Taskinen MR, Wahlstrand B, Hughes TE, Parnell LD, Lai CQ, Berglund G, Peltonen L, Vartiainen E, Jousilahti P, Havulinna AS, Salomaa V, Nilsson P, Groop L, Altshuler D, Ordovas JM, Kathiresan S (2008) Common missense variant in the glucokinase regulatory protein gene is associated with increased plasma triglyceride and C-reactive protein but lower fasting glucose concentrations. Diabetes 57(11):3112–3121. https://doi.org/10.2337/db08-0516
Vaxillaire M, Cavalcanti-Proenca C, Dechaume A, Tichet J, Marre M, Balkau B, Froguel P, Group DS (2008) The common P446L polymorphism in GCKR inversely modulates fasting glucose and triglyceride levels and reduces type 2 diabetes risk in the DESIR prospective general French population. Diabetes 57(8):2253–2257. https://doi.org/10.2337/db07-1807
Bi M, Kao WH, Boerwinkle E, Hoogeveen RC, Rasmussen-Torvik LJ, Astor BC, North KE, Coresh J, Kottgen A (2010) Association of rs780094 in GCKR with metabolic traits and incident diabetes and cardiovascular disease: the ARIC Study. PLoS One 5(7):e11690. https://doi.org/10.1371/journal.pone.0011690
Simons N, Dekker JM, van Greevenbroek MM, Nijpels G, t Hart LM, van der Kallen CJ, Schalkwijk CG, Schaper NC, Stehouwer CD, Brouwers MC (2016) A common gene variant in glucokinase regulatory protein interacts with glucose metabolism on diabetic dyslipidemia: the combined CODAM and Hoorn studies. Diabetes Care 39(10):1811–1817. https://doi.org/10.2337/dc16-0153
Simons P, Simons N, Stehouwer CDA, Schalkwijk CG, Schaper NC, Brouwers M (2018) Association of common gene variants in glucokinase regulatory protein with cardiorenal disease: a systematic review and meta-analysis. PLoS One 13(10):e0206174. https://doi.org/10.1371/journal.pone.0206174
Bray GA, Nielsen SJ, Popkin BM (2004) Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 79(4):537–543. https://doi.org/10.1093/ajcn/79.4.537
Bray GA (2008) Fructose: should we worry? Int J Obes (Lond) 32(Suppl 7):S127–S131. https://doi.org/10.1038/ijo.2008.248
Stanhope KL, Havel PJ (2008) Fructose consumption: potential mechanisms for its effects to increase visceral adiposity and induce dyslipidemia and insulin resistance. Curr Opin Lipidol 19(1):16–24. https://doi.org/10.1097/MOL.0b013e3282f2b24a
Lustig RH (2010) Fructose: metabolic, hedonic, and societal parallels with ethanol. J Am Diet Assoc 110(9):1307–1321. https://doi.org/10.1016/j.jada.2010.06.008
Ludwig J, Viggiano TR, McGill DB, Oh BJ (1980) Nonalcoholic steatohepatitis: mayo clinic experiences with a hitherto unnamed disease. Mayo Clin Proc 55(7):434–438
Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C (2016) Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol 65(3):589–600. https://doi.org/10.1016/j.jhep.2016.05.013
Mantovani A, Byrne CD, Bonora E, Targher G (2018) Nonalcoholic fatty liver disease and risk of incident type 2 diabetes: a meta-analysis. Diabetes Care 41(2):372–382. https://doi.org/10.2337/dc17-1902
Stender S, Kozlitina J, Nordestgaard BG, Tybjaerg-Hansen A, Hobbs HH, Cohen JC (2017) Adiposity amplifies the genetic risk of fatty liver disease conferred by multiple loci. Nat Genet 49(6):842–847. https://doi.org/10.1038/ng.3855
Zhang L, Perdomo G, Kim DH, Qu S, Ringquist S, Trucco M, Dong HH (2008) Proteomic analysis of fructose-induced fatty liver in hamsters. Metabolism 57(8):1115–1124. https://doi.org/10.1016/j.metabol.2008.03.017
Mock K, Lateef S, Benedito VA, Tou JC (2017) High-fructose corn syrup-55 consumption alters hepatic lipid metabolism and promotes triglyceride accumulation. J Nutr Biochem 39:32–39. https://doi.org/10.1016/j.jnutbio.2016.09.010
Schultz A, Barbosa-da-Silva S, Aguila MB, Mandarim-de-Lacerda CA (2015) Differences and similarities in hepatic lipogenesis, gluconeogenesis and oxidative imbalance in mice fed diets rich in fructose or sucrose. Food Funct 6(5):1684–1691. https://doi.org/10.1039/c5fo00251f
Ackerman Z, Oron-Herman M, Grozovski M, Rosenthal T, Pappo O, Link G, Sela BA (2005) Fructose-induced fatty liver disease: hepatic effects of blood pressure and plasma triglyceride reduction. Hypertension 45(5):1012–1018. https://doi.org/10.1161/01.HYP.0000164570.20420.67
Chiu S, Sievenpiper JL, de Souza RJ, Cozma AI, Mirrahimi A, Carleton AJ, Ha V, Di Buono M, Jenkins AL, Leiter LA, Wolever TM, Don-Wauchope AC, Beyene J, Kendall CW, Jenkins DJ (2014) Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of controlled feeding trials. Eur J Clin Nutr 68(4):416–423. https://doi.org/10.1038/ejcn.2014.8
Perez-Pozo SE, Schold J, Nakagawa T, Sanchez-Lozada LG, Johnson RJ, Lillo JL (2010) Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: role of uric acid in the hypertensive response. Int J Obes (Lond) 34(3):454–461. https://doi.org/10.1038/ijo.2009.259
Fox IH, Kelley WN (1972) Studies on the mechanism of fructose-induced hyperuricemia in man. Metabolism 21(8):713–721. https://doi.org/10.1016/0026-0495(72)90120-5
Hurtado del Pozo C, Vesperinas-Garcia G, Rubio MA, Corripio-Sanchez R, Torres-Garcia AJ, Obregon MJ, Calvo RM (2011) ChREBP expression in the liver, adipose tissue and differentiated preadipocytes in human obesity. Biochim Biophys Acta 1811(12):1194–1200. https://doi.org/10.1016/j.bbalip.2011.07.016
Petersen KF, Laurent D, Yu C, Cline GW, Shulman GI (2001) Stimulating effects of low-dose fructose on insulin-stimulated hepatic glycogen synthesis in humans. Diabetes 50(6):1263–1268. https://doi.org/10.2337/diabetes.50.6.1263
Softic S, Gupta MK, Wang GX, Fujisaka S, O’Neill BT, Rao TN, Willoughby J, Harbison C, Fitzgerald K, Ilkayeva O, Newgard CB, Cohen DE, Kahn CR (2017) Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J Clin Invest 127(11):4059–4074. https://doi.org/10.1172/JCI94585
Miller C, Yang X, Lu K, Cao J, Herath K, Rosahl TW, Askew R, Pavlovic G, Zhou G, Li C, Akiyama TE (2018) Ketohexokinase knockout mice, a model for essential fructosuria, exhibit altered fructose metabolism and are protected from diet-induced metabolic defects. Am J Physiol Endocrinol Metab. https://doi.org/10.1152/ajpendo.00027.2018
Ishimoto T, Lanaspa MA, Rivard CJ, Roncal-Jimenez CA, Orlicky DJ, Cicerchi C, McMahan RH, Abdelmalek MF, Rosen HR, Jackman MR, MacLean PS, Diggle CP, Asipu A, Inaba S, Kosugi T, Sato W, Maruyama S, Sanchez-Lozada LG, Sautin YY, Hill JO, Bonthron DT, Johnson RJ (2013) High-fat and high-sucrose (western) diet induces steatohepatitis that is dependent on fructokinase. Hepatology 58(5):1632–1643. https://doi.org/10.1002/hep.26594
Ishimoto T, Lanaspa MA, Le MT, Garcia GE, Diggle CP, Maclean PS, Jackman MR, Asipu A, Roncal-Jimenez CA, Kosugi T, Rivard CJ, Maruyama S, Rodriguez-Iturbe B, Sanchez-Lozada LG, Bonthron DT, Sautin YY, Johnson RJ (2012) Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proc Natl Acad Sci USA 109(11):4320–4325. https://doi.org/10.1073/pnas.1119908109
Huard K, Ahn K, Amor P, Beebe DA, Borzilleri KA, Chrunyk BA, Coffey SB, Cong Y, Conn EL, Culp JS, Dowling MS, Gorgoglione MF, Gutierrez JA, Knafels JD, Lachapelle EA, Pandit J, Parris KD, Perez S, Pfefferkorn JA, Price DA, Raymer B, Ross TT, Shavnya A, Smith AC, Subashi TA, Tesz GJ, Thuma BA, Tu M, Weaver JD, Weng Y, Withka JM, Xing G, Magee TV (2017) Discovery of fragment-derived small molecules for in vivo inhibition of ketohexokinase (KHK). J Med Chem 60(18):7835–7849. https://doi.org/10.1021/acs.jmedchem.7b00947
Speliotes EK, Yerges-Armstrong LM, Wu J, Hernaez R, Kim LJ, Palmer CD, Gudnason V, Eiriksdottir G, Garcia ME, Launer LJ, Nalls MA, Clark JM, Mitchell BD, Shuldiner AR, Butler JL, Tomas M, Hoffmann U, Hwang SJ, Massaro JM, O’Donnell CJ, Sahani DV, Salomaa V, Schadt EE, Schwartz SM, Siscovick DS, Nash CRN, Consortium G, Investigators M, Voight BF, Carr JJ, Feitosa MF, Harris TB, Fox CS, Smith AV, Kao WH, Hirschhorn JN, Borecki IB, Consortium G (2011) Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet 73:e1001324. https://doi.org/10.1371/journal.pgen.1001324
Seedorf U, Sen-Chowdhry S, Siminovitch KA, Smit JH, Spector TD, Tan W, Teslovich TM, Tukiainen T, Uitterlinden AG, Van der Klauw MM, Vasan RS, Wallace C, Wallaschofski H, Wichmann HE, Willemsen G, Wurtz P, Xu C, Yerges-Armstrong LM, Alcohol Genome-wide Association C, Diabetes Genetics R, Meta-analyses S, Genetic Investigation of Anthropometric Traits C, Global Lipids Genetics C, Genetics of Liver Disease C, International Consortium for Blood P, Meta-analyses of G, Insulin-Related Traits C, Abecasis GR, Ahmadi KR, Boomsma DI, Caulfield M, Cookson WO, van Duijn CM, Froguel P, Matsuda K, McCarthy MI, Meisinger C, Mooser V, Pietilainen KH, Schumann G, Snieder H, Sternberg MJ, Stolk RP, Thomas HC, Thorsteinsdottir U, Uda M, Waeber G, Wareham NJ, Waterworth DM, Watkins H, Whitfield JB, Witteman JC, Wolffenbuttel BH, Fox CS, Ala-Korpela M, Stefansson K, Vollenweider P, Volzke H, Schadt EE, Scott J, Jarvelin MR, Elliott P, Kooner JS (2011) Genome-wide association study identifies loci influencing concentrations of liver enzymes in plasma. Nat Genet 43(11):1131–1138. https://doi.org/10.1038/ng.970
van der Harst P, Bakker SJ, de Boer RA, Wolffenbuttel BH, Johnson T, Caulfield MJ, Navis G (2010) Replication of the five novel loci for uric acid concentrations and potential mediating mechanisms. Hum Mol Genet 19(2):387–395. https://doi.org/10.1093/hmg/ddp489
Kolz M, Johnson T, Sanna S, Teumer A, Vitart V, Perola M, Mangino M, Albrecht E, Wallace C, Farrall M, Johansson A, Nyholt DR, Aulchenko Y, Beckmann JS, Bergmann S, Bochud M, Brown M, Campbell H, Consortium E, Connell J, Dominiczak A, Homuth G, Lamina C, McCarthy MI, Consortium E, Meitinger T, Mooser V, Munroe P, Nauck M, Peden J, Prokisch H, Salo P, Salomaa V, Samani NJ, Schlessinger D, Uda M, Volker U, Waeber G, Waterworth D, Wang-Sattler R, Wright AF, Adamski J, Whitfield JB, Gyllensten U, Wilson JF, Rudan I, Pramstaller P, Watkins H, Consortium P, Doering A, Wichmann HE, Study K, Spector TD, Peltonen L, Volzke H, Nagaraja R, Vollenweider P, Caulfield M, Wtccc Illig T, Gieger C (2009) Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet 5(6):e1000504. https://doi.org/10.1371/journal.pgen.1000504
Acknowledgements
The study in HFI patients was subsidized by the Netherlands Heart Foundation (#2015T042) and Stofwisselkracht. MB received a personal grant from the Diabetes Foundation (#2017.82.004). DT was supported by Colorado Research Partners LLC and National Institutes of Health (R01DK108859).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Buziau, A.M., Schalkwijk, C.G., Stehouwer, C.D. et al. Recent advances in the pathogenesis of hereditary fructose intolerance: implications for its treatment and the understanding of fructose-induced non-alcoholic fatty liver disease. Cell. Mol. Life Sci. 77, 1709–1719 (2020). https://doi.org/10.1007/s00018-019-03348-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03348-2