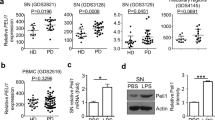
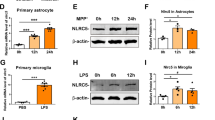
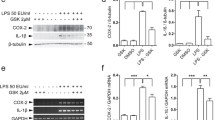
Abstract
Parkinson’s disease (PD) is characterized by a progressive loss of dopaminergic neurons in the substantia nigra. The cause of neuronal death in PD is largely unknown, but several genetic loci, including PTEN-induced putative kinase 1 (PINK1), have been linked to early onset autosomal recessive forms of familial PD. PINK1 encodes a serine/threonine kinase, which phosphorylates several substrates and consequently leads to cell protection against apoptosis induced by various stresses. In addition, research has shown that inflammation largely contributes to the pathogenesis of PD, but the functional link between PINK1 and PD-linked neuroinflammation remains poorly understood. Therefore, in the present study, we investigated the functional role of PINK1 in interleukin (IL)-1β-mediated inflammatory signaling. We show that PINK1 specifically binds to TRAF6 and TAK1, and facilitates the autodimerization and autoubiquitination of TRAF6. PINK1 also enhances the association between TRAF6 and TAK1, phosphorylates TAK1, and stimulates polyubiquitination of TAK1. Furthermore, PINK1 leads to the potentiation of IL-1β-mediated NF-κB activity and cytokine production. These findings suggest that PINK1 positively regulates two key molecules, TRAF6 and TAK1, in the IL-1β-mediated signaling pathway, consequently up-regulating their downstream inflammatory events.









Similar content being viewed by others
Abbreviations
- DAPI:
-
4, 6-Diamidino-2-phenylindole
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- ECL:
-
Enhanced chemiluminescence
- FBS:
-
Fetal bovine serum
- HEK293:
-
Human embryonic kidney 293
- IKK:
-
IκB kinase
- IL-1:
-
Interleukin-1
- IL-1R:
-
IL-1 receptor
- IRAK1:
-
IL-1R-associated kinase-1
- LPS:
-
Lipopolysaccharide
- MEFs:
-
Mouse embryonic fibroblasts
- Myd88:
-
Myeloid differentiation marker
- NF-κB:
-
Nuclear factor-kappa B
- NO:
-
Nitric oxide
- PD:
-
Parkinson’s disease
- PINK1:
-
PTEN-induced putative kinase 1
- ROS:
-
Reactive oxygen species
- TAK1:
-
TGF-β activated kinase-1
- TGF-β:
-
Transforming growth factor-β
- TNF:
-
Tumor necrosis factor
- TRAF6:
-
TNF receptor-associated factor 6
- GST:
-
Glutathion S-transferase
References
Olanow CW, Tatton WG (1999) Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci 22:123–144
Moore DJ, West AB, Dawson VL, Dawson TM (2005) Molecular pathophysiology of Parkinson’s disease. Annu Rev Neurosci 28:57–87
Silvestri L, Caputo V, Bellacchio E, Atorino L, Dallapiccola B, Valente EM, Casari G (2005) Mitochondrial import and enzymatic activity of PINK1 mutants associated to recessive parkinsonism. Hum Mol Genet 15:3477–3492
Beilina A, Van Der Brug M, Ahmad R, Kesavapany S, Miller DW, Petsko GA, Cookson MR (2005) Mutations in PTEN-induced putative kinase 1 associated with recessive parkinsonism have differential effects on protein stability. Proc Natl Acad Sci USA 19:5703–5708
Pridgeon JW, Olzmann JA, Chin LS, Li L (2007) PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol 5:e172
Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, González-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ (2004) Hereditary early onset Parkinson’s disease caused by mutations in PINK1. Science 21:1158–1160
Petit A, Kawarai T, Paitel E, Sanjo N, Maj M, Scheid M, Chen F, Gu Y, Hasegawa H, Salehi-Rad S, Wang L, Rogaeva E, Fraser P, Robinson B, St George-Hyslop P, Tandon A (2005) Wild-type PINK1 prevents basal and induced neuronal apoptosis, a protective effect abrogated by Parkinson disease-related mutations. J Biol Chem 7:34025–34032
Kaltschmidt B, Widera D, Kaltschmidt C (2005) Signaling via NF-kappaB in the nervous system. Biochim Biophys Acta 30:287–299
Aloisi F (2001) Immune function of microglia. Glia 36:165–179
Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, Nagatsu T (1996) Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neurosci Lett 211:13–16
Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T (1994) Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from Parkinsonian patients. Neurosci Lett 165:208–210
Arend WP (2002) The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev 13:323–340
Janssens S, Burns K, Tschopp J, Beyaert R (2002) Regulation of interleukin-1- and lipopolysaccharide-induced NF-kappaB activation by alternative splicing of MyD88. Curr Biol 19:467–471
Gan L, Li L (2006) Regulations and roles of the interleukin-1 receptor associated kinases (IRAKs) in innate and adaptive immunity. Immunol Res 35:295–302
Inoue J, Gohda J, Akiyama T (2007) Characteristics and biological functions of TRAF6. Adv Exp Med Biol 597:72–79
Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ (2001) TAK1 is an ubiquitin-dependent kinase of MKK and IKK. Nature 19:346–351
Martin MU, Wesche H (2002) Summary and comparison of the signaling mechanisms of the Toll/interleukin-1 receptor family. Biochim Biophys Acta 11:265–280
Huang Q, Yang J, Lin Y, Walker C, Cheng J, Liu ZG, Su B (2004) Differential regulation of interleukin 1 receptor and Toll-like receptor signaling by MEKK3. Nat Immunol 5:98–103
Su J, Richter K, Zhang C, Gu Q, Li L (2007) Differential regulation of interleukin-1 receptor associated kinase 1 (IRAK1) splice variants. Mol Immunol 44:900–905
Um JW, Stichel-Gunkel C, Lübbert H, Lee G, Chung KC (2009) Molecular interaction between parkin and PINK1 in mammalian neuronal cells. Mol Cell Neurosci 40:421–432
Kishimoto K, Matsumoto K, Ninomiya-Tsuji J (2000) TAK1 mitogen-activated protein kinase kinase kinase is activated by autophosphorylation within its activation loop. J Biol Chem 10:7359–7364
Cao Z, Henzel WJ, Gao X (1996) IRAK: a kinase associated with the interleukin-1 receptor. Science 23:1128–1131
Deng L, Wang C, Spencer E, Yang L, Braun A, You J, Slaughter C, Pickart C, Chen ZJ (2000) Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 13:351–361
Hayden MS, Ghosh S (2004) Signaling to NF-kappaB. Genes Dev 15:2195–2224
Fan Y, Yu Y, Shi Y, Sun W, Xie M, Ge N, Mao R, Chang A, Xu G, Schneider MD, Zhang H, Fu S, Qin J, Yang J (2010) Lysine 63-linked polyubiquitination of TAK1 at lysine 158 is required for tumor necrosis factor alpha- and interleukin-1beta-induced IKK/NF-kappaB and JNK/AP-1 activation. J Biol Chem 19:5347–5360
Yamazaki K, Gohda J, Kanayama A, Miyamoto Y, Sakurai H, Yamamoto M, Akira S, Hayashi H, Su B, Inoue J (2009) Two mechanistically and temporally distinct NF-kappaB activation pathways in IL-1 signaling. Sci Signal 20:ra66
Kajino T, Ren H, Iemura S, Natsume T, Stefansson B, Brautigan DL, Matsumoto K, Ninomiya-Tsuji J (2006) Protein phosphatase 6 down-regulates TAK1 kinase activation in the IL-1 signaling pathway. J Biol Chem 29:39891–39896
Yu Y, Ge N, Xie M, Sun W, Burlingame S, Pass AK, Nuchtern JG, Zhang D, Fu S, Schneider MD, Fan J, Yang J (2008) Phosphorylation of Thr-178 and Thr-184 in the TAK1 T-loop is required for interleukin (IL)-1-mediated optimal NF-kappaB and AP-1 activation as well as IL-6 gene expression. J Biol Chem 5:24497–24505
Hisanaga K, Asagi M, Itoyama Y, Iwasaki Y (2001) Increase in peripheral CD4 bright + CD8 dull + T cells in Parkinson disease. Arch Neurol 58:1580–1583
Bas J, Calopa M, Mestre M, Molleví DG, Cutillas B, Ambrosio S, Buendia E (2001) Lymphocyte populations in Parkinson’s disease and in rat models of parkinsonism. J Neuroimmunol 113:146–152
Wu Q, Combs C, Cannady SB, Geldmacher DS, Herrup K (2000) Beta-amyloid activated microglia induce cell cycling and cell death in cultured cortical neurons. Neurobiol Aging 21:797–806
Castano A, Herrera AJ, Cano J, Machado A (1998) Lipopolysaccha-ride intranigral injection induces inflammatory reaction and damage in nigrostriatal dopaminergic system. J Neurochem 70:1584–1592
Frank-Cannon TC, Tran T, Ruhn KA, Martinez TN, Hong J, Marvin M, Hartley M, Treviño I, O’Brien DE, Casey B, Goldberg MS, Tansey MG (2008) Parkin deficiency increases vulnerability to inflammation-related nigral degeneration. J Neurosci 28:10825–10834
Sung JY, Park SM, Lee CH, Um JW, Lee HJ, Kim J, Oh YJ, Lee ST, Paik SR, Chung KC (2005) Proteolytic cleavage of extracellular secreted alpha-synuclein via matrix metalloproteinases. J Biol Chem 280:25216–25224
Park JY, Kim KS, Lee SB, Ryu JS, Chung KC, Choo YK, Jou I, Kim J, Park SM (2009) On the mechanism of internalization of alpha-synuclein into microglia: roles of ganglioside GM1 and lipid raft. J Neurochem 110:400–411
Verstrepen L, Bekaert T, Chau TL, Tavernier J, Chariot A, Beyaert R (2008) TLR-4, IL-1R and TNF-R signaling to NF-kappaB: variations on a common theme. Cell Mol Life Sci 65:2964–2978
Akundi RS, Huang Z, Eason J, Pandya JD, Zhi L, Cass WA, Sullivan PG, Büeler H (2011) Increased mitochondrial calcium sensitivity and abnormal expression of innate immunity genes precede dopaminergic defects in Pink1-deficient mice. PLoS ONE 6:e16038
Sim CH, Lio DS, Mok SS, Masters CL, Hill AF, Culvenor JG, Cheng HC (2006) C-terminal truncation and Parkinson’s disease-associated mutations down-regulate the protein serine/threonine kinase activity of PTEN-induced kinase-1. Hum Mol Genet 15:3251–3262
Weihofen A, Ostaszewski B, Minami Y, Selkoe DJ (2008) Pink1 Parkinson mutations, the Cdc37/Hsp90 chaperones and Parkin all influence the maturation or subcellular distribution of Pink1. Hum Mol Genet 17:602–616
Strauss KM, Martins LM, Plun-Favreau H, Marx FP, Kautzmann S, Berg D, Gasser T, Wszolek Z, Müller T, Bornemann A, Wolburg H, Downward J, Riess O, Schulz JB, Krüger R (2005) Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson’s disease. Hum Mol Genet 14:2099–2111
Kim Y, Park J, Kim S, Song S, Kwon SK, Lee SH, Kitada T, Kim JM, Chung J (2008) PINK1 controls mitochondrial localization of Parkin through direct phosphorylation. Biochem Biophys Res Commun 377:975–980
Narendra D, Tanaka A, Suen DF, Youle RJ (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183:795–803
Um JW, Park HJ, Song J, Jeon I, Lee G, Lee PH, Chung KC (2010) Formation of parkin aggregates and enhanced PINK1 accumulation during the pathogenesis of Parkinson’s disease. Biochem Biophys Res Commun 393:824–828
Takatori S, Ito G, Iwatsubo T (2008) Cytoplasmic localization and proteasomal degradation of N-terminally cleaved form of PINK1. Neurosci Lett 430:13–17
Lin W, Kang UJ (2008) Characterization of PINK1 processing, stability, and subcellular localization. J Neurochem 106:464–474
Xiong H, Wang D, Chen L, Choo YS, Ma H, Tang C, Xia K, Jiang W, Ronai Z, Zhuang X, Zhang Z (2009) Parkin, PINK1, and DJ-1 form a ubiquitin E3 ligase complex promoting unfolded protein degradation. J Clin Invest 119:650–660
Murata H, Sakaguchi M, Jin Y, Sakaguchi Y, Futami J, Yamada H, Kataoka K, Huh NH (2011) A new cytosolic pathway from a Parkinson disease-associated kinase, BRPK/PINK1: activation of AKT via mTORC2. J Biol Chem 286:7182–7189
Adhikari A, Xu M, Chen ZJ (2007) Ubiquitin-mediated activation of TAK1 and IKK. Oncogene 14:3214–3226
Inagaki M, Omori E, Kim JY, Komatsu Y, Scott G, Ray MK, Yamada G, Matsumoto K, Mishina Y, Ninomiya-Tsuji J (2008) TAK1-binding protein 1, TAB 1, mediates osmotic stress-induced TAK1 activation but is dispensable for TAK1-mediated cytokine signaling. J Biol Chem 283:33080–33086
Acknowledgments
We thank J. Chung and G. Takaesu for providing plasmids. We are also grateful to G. Takaesu for providing 293 IL-1RI cells and to J. Shen for PINK1−/− and +/+ MEFs. This study was supported by grants from the Brain Research Center of the 21st Century Frontier Research Program Technology (2009 K-001251 to K.C.C.) and from the Korea Science and Engineering Foundation (KOSEF) through the National Research Laboratory Program (2010-0018916 to K.C.C.) funded by the Ministry of Education, Science and Technology (MEST) of the Republic of Korea. This work was also partly supported by grants from KOSEF (2012-0000810 to K.C.C.) and from the Korea Health 21 R&D Project (A092004 and A111653 to K.C.C.), Ministry of Health, Welfare and Family Affairs.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, H.J., Jang, S.H., Kim, H. et al. PINK1 stimulates interleukin-1β-mediated inflammatory signaling via the positive regulation of TRAF6 and TAK1. Cell. Mol. Life Sci. 69, 3301–3315 (2012). https://doi.org/10.1007/s00018-012-1004-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1004-7