Abstract
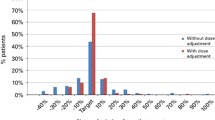
Maintaining the appropriate average steady-state plasma concentrations (Css) of busulfan (BU) is critical for both successful engraftment and minimizing toxicity in hematopoietic stem cell transplantation (HST). We therefore performed a prospective trial with 50 adult Japanese patients that involved adjusting the BU dose in accordance with individual BU phar-macokinetics (PK). After administering a 0.5-mg/kg test dose of oral BU, we analyzed individual BU PK parameters and calculated an adjusted BU dose that would achieve a target BU Css of 850 ng/mL. Thirty-nine patients (78%) required a BU dose decrease, and the median adjusted BU dose was 0.81 mg/kg (range, 0.51–1.29 mg/kg). All patients who underwent allogeneic HST received the adjusted BU dose. After administering the sixth BU dose, we measured the plasma BU concentration. The actual BU concentration was significantly correlated with the expected BU concentration, and the predictability of the BU Css was 103% ± 19%. The incidence of toxicity excluding oral mucositis was low, and there was no regimen-related toxicity-associated mortality. Engraftment was achieved in 98% of the patients. This study showed that our method for adjusting the BU dose facilitated reliable prediction of the actual BU Css and that individualized BU dose adjustment was able to improve clinical outcomes in HST recipients treated with a BU-containing conditioning regimen.
Similar content being viewed by others
References
Hassan M, Ljungman P, Bolme P, et al. Busulfan bioavailability. Blood. 1994;84:2144–2150.
Dix SP, Wingard JR, Mullins RE, et al. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant. 1996;17:225–230.
Slattery JT, Clift RA, Buckner CD, et al. Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood. 1997;89:3055–3060.
Bolinger AM, Zangwill AB, Slattery JT, et al. An evaluation of engraftment, toxicity and busulfan concentration in children receiving bone marrow transplantation for leukemia or genetic disease. Bone Marrow Transplant. 2000;25:925–930.
McCune JS, Gibbs JP, Slattery JT. Plasma concentration monitoring of busulfan: does it improve clinical outcome? Clin Pharmacokinet. 2000;39:155–165.
Deeg HJ, Storer B, Slattery JT, et al. Conditioning with targeted busulfan and cyclophosphamide for hemopoietic stem cell transplantation from related and unrelated donors in patients with myelodysplastic syndrome. Blood. 2002;100:1201–1207.
Radich JP, Gooley T, Bensinger W, et al. HLA-matched related hematopoietic cell transplantation for chronic-phase CML using a targeted busulfan and cyclophosphamide preparative regimen. Blood. 2003;102:31–35.
Takamatsu Y, Ogata K, Yamauchi K, et al. An evaluation of busulfan pharmacokinetics in patients undergoing hematopoietic stem cell transplantation. Jpn J Clin Oncol. 2005;35:400–403.
Hara S, Tsuchie M, Tsujioka R, et al. High-performance liquid chromatographic quantification of busulfan in human serum after fluorescence derivatization by 2-naphthalenethiol. Anal Sci. 2000;16:287–291.
Sandstrom M, Karlsson MO, Ljungman P, et al. Population pharmacokinetic analysis resulting in a tool for dose individualization of busulfan in bone marrow transplantation recipients. Bone Marrow Transplant. 2001;28:657–664.
Higuchi S, Aoyama T, Horioka M PEDA: a microcomputer program for parameter estimation and dosage adjustment in clinical practice. J Pharmacobiodyn. 1987;10:703–718.
Bearman SI, Appelbaum FR, Buckner CD, et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol. 1988;6:1562–1568.
Jones RJ, Lee KS, Beschorner WE, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987:778–783.
McCune JS, Gooley T, Gibbs JP, et al. Busulfan concentration and graft rejection in pediatric patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;30:167–173.
Bleyzac N, Souillet G, Magron P, et al. Improved clinical outcome of paediatric bone marrow recipients using a test dose and Bayesian pharmacokinetic individualization of busulfan dosage regimens. Bone Marrow Transplant. 2001;28:743–751.
Hassan M, Oberg G, Bjorkholm M,Wallin I, Lindgren M. Influence of prophylactic anticonvulsant therapy on high-dose busulphan kinetics. Cancer Chemother Pharmacol. 1993;33:181–186.
Buggia I, Zecca M, Alessandrino EP, et al. Itraconazole can increase systemic exposure to busulfan in patients given bone marrow transplantation. Anticancer Res. 1996;16:2083–2088.
Nilsson C, Aschan J, Hentschke P, Ringden O, Ljungman P, Hassan M. The effect of metronidazole on busulfan pharmacokinetics in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2003;31:429–435.
Bornhauser M, Storer B, Slattery JT, et al. Conditioning with fludarabine and targeted busulfan for transplantation of allogeneic hematopoietic stem cells. Blood. 2003;102:820–826.
Hansen JA, Petersdorf EW. Unrelated donor hematopoietic cell transplantation. In: Thomas ED, Blume KG, Forman SJ, eds. Hematopoietic Cell Transplantation. 2nd ed. Malden, Mass: Blackwell Science; 1999:915–928.
Bolinger AM, Zangwill AB, Slattery JT, et al. Target dose adjustment of busulfan in pediatric patients undergoing bone marrow transplantation. Bone Marrow Transplant. 2001;28:1013–1018.
Poonkuzhali B, Chandy M, Srivastava A, Dennison D, Krishnamoorthy R. Glutathione S-transferase activity influences busulfan pharmacokinetics in patients with beta thalassemia major undergoing bone marrow transplantation. Drug Metab Dispos. 2001;29:264–267.
Srivastava A, Poonkuzhali B, Shaji RV, et al. Glutathione S-transferase M1 polymorphism: a risk factor for hepatic venoocclusive disease in bone marrow transplantation. Blood. 2004;104:1574–1577.
Nelson HH, Wiencke JK, Christiani DC, et al. Ethnic differences in the prevalence of the homozygous deleted genotype of glutathione S-transferase theta. Carcinogenesis. 1995;16:1243–1245.
Takama H,Tanaka H, Nakashima D, Ueda R, Takaue Y. Population pharmacokinetics of intravenous busulfan in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2006;37:345–351.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Takamatsu, Y., Sasaki, N., Eto, T. et al. Individual Dose Adjustment of Oral Busulfan Using a Test Dose in Hematopoietic Stem Cell Transplantation. Int J Hematol 86, 261–268 (2007). https://doi.org/10.1532/IJH97.07013
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1532/IJH97.07013