Abstract
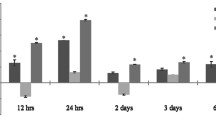
• Background: After unilateral anterior chamber (AC) inoculation with herpes simplex virus type 1 (HSV-1), C.B-17 and BALB/c congenic mice, which differ only in a limited region around the Igh-1 locus on chromosome 12, show a striking difference in susceptibility to development of encephalitis and contralateral necrotizing chorioretinitis. • Methods: After AC inoculation with HSV-1 (KOS), C.B-17 and BALB/c mice were followed up for the clinical signs of encephalitis and chorioretinitis. At different time points following inoculation, lymphocytes isolated from the spleen were triple-stained with antibodies directed against CD4 or CD8, IL-2R, and various V β T-cell receptor (TCR) subsets, and were analyzed by flow cytometry. • Results: These Igh-l-disparate congenic mice showed differences in the time course of splenic V β T-cell receptor (TCR) usage in both CD4+, IL-2R+ and CD8+, IL-2R+ T cells. By day 1 post infection (p.i.), C.B-17 mice showed an increase of V β 8 and V β 9 TCR by both CD4+, IL-2R+ and CD8+, IL-2R+ splenic T cells. Susceptible BALB/c mice delayed the increase of splenic V β 8 and V β 9 TCR by CD4+, IL-2R+ T cells, which was noted by day 4 p.i. Furthermore, in BALB/c mice the usage of V β 9 by CD8+ cells was increased by day 6 p.i. • Conclusions: Our findings indicate that early preferential splenic usage of a restricted repertoire of TCR occurs after ocular inoculation with HSV-1 in resistant C.B-17 mice. Such preferential TCR usage by activated T cells may prevent viral replication in the brain and contralateral eye and may be linked to protection from development of encephalitis and destructive herpes-mediated ocular inflammation.
Similar content being viewed by others
References
Aebisher T, Oehen S, Hengartner H (1990) Preferential usage of Va4 and Vb10 T cell receptor genes by lymphocyte choriomeningitis virus glycoprotein-specific H-2Dd-restricted cytotoxic T cells. Eur J Immunol 20:253–531
Atherton SS, Altman NH, Streilein JW (1989) Histopathologic study of herpes virus-induced retinitis in athymic mice: evidence for an immunopathogenic process. Curr Eye Res 8:1179–1192
Bonneau RH, Jennings SR (1989) Modulation of acute and latent herpes simplex virus infection in C57BL/6 mice by adoptive transfer of immune lymphocytes with cytotoxic activity. J Virol 63:1480–1484
Bosem ME, Harris R, Atherton SS (1990) Optic nerve involvement in viral spread in herpes simplex type 1 retinitis. Invest Ophthalmol Vis Sci 31:1683–1689
Cousins SW, Gonzalez A, Atherton SS (1989) Herpes simplex retinitis in the mouse: clinicopathologic correlations. Invest Ophthalmol Vis Sci 30:1485–1494
Culbertson WW, Blumenkranz MS, Pepose JS, Stewart JA, Curtin VT (1986) Varicella zoster virus is a cause of acute retinal necrosis syndrome. Ophthalmology 93:559–569
Foster CS, Opremcak EM, Rice B, Wells P, Chung H, Thompson P, Phong LP, Raizman M (1987) Clinical, pathologic, and immunopathologic characteristics of experimental murine herpes simplex virus stromal keratitis and uveitis is controlled by gene products from the igh-1 locus on chromosome 12. Trans Am Ophthalmol Soc 85:293–311
Foster CS, Zierhut M, Wu H, Tamesis R, Jabbur N (1991) Murine acute retinal necrosis. Trans Am Ophthalmol Soc 89:251–268
Hemady R, Tauber J, Ihley TM, Opremcak EM, Foster CS (1990) Viral isolation and systemic immune responses after intracameral inoculation of herpes simplex virus type 1 in Igh-1 disparate congenic murine strains. Invest Ophthalmol Vis Sci 31:2335–2341
Igietseme JU, Streilein JW, Miranda F, Feinerman SJ, Atherton SS (1991) Mechanisms of protection against herpes simplex type-1 induced retinal necrosis by in vitro-activated T lymphocytes. J Virol 65:763–768
Kaplan HJ, Streilein JW (1974) Do immunologically privileged sites require a functioning spleen? Nature 251:553–554
Larsen HS, Russel RG, Rouse BT (1983) Recovery from lethal herpes simplex virus type 1 infection is mediated by cytotoxic T lymphocytes. Infect Immun 27:133–139
Lewis ML, Culbertson WW, Post MJD, Miller D, Kokame GT, Dix RD (1989) Herpes simples virus type 1. A cause of the acute retinal necrosis syndrome. Ophthalmology 96: 875–878
Owen FL, Riblet R (1984) Genes for the mouse T cell alloantigens Tpre, TThy, tind and tsu are closely near Igh on chromosome 12. J Exp Med 159:313–329
Owen FL, Ju ST, Nisonoff A (1977) Binding of idiotypic determinants of large proportions of thymus derived lymphocytes in idiotypically sup pressed mice. Proc Natl Acad Sci USA 74:2084–2088
Rodewald HR, Koszinowsky UH, Eichmann K, Melchers I (1989) Predominant utilization of Vb8+ T cell receptor genes in the H-2Ld-restricted cytotoxic T cell response against the immediate-early protein pp89 of the murine cytomegalovirus. J Immunol 143:4238–4243
Rodriguez M, Patick AK, Pease LR, David CS (1992) Role of T cell receptor V β genes in Theiler's virus-induced demyelination of mice. J Immunol 148:921–927
Sandstrom IK, Foster CS, Wells PA, Knipe D, Caron L, Greene MI (1986) Previous immunization of mice with herpes simplex virus type-1 strain MP protects against secondary corneal infection. Clin Immunol Immunopathol 40:326–333
Sethi KK, Omata Y, Schneweis (1983) Protection of mice from fatal HSV-1 infection by adoptive transfer of cloned virus-specific cytotoxic T lymphocytes. J Gen Virol 64:443–447
Streilein JW (1987) Immune regulation and the eye: a dangerous compromise. FASEB 1:199–208
Streilein JW, Niederkorn JY (1981) Induction of anterior chamber-associated immune deviation requires an intact, functional spleen. J Exp Med 153:1058–1063
Streilein JW, Atherton SS, Vann VR (1987) A critical role for ACAID in the distinctive pattern of retinitis that follows anterior chamber inoculation. Curr Eye Res 6:127–131
Szily A v (1924) Experimentelle endogene Infektionen. Übertragung von Bulbus zu Bulbus. Klin Monatsbl Augenheilkd 72:593–602
Vann VR, Atherton SS (1991) Neural spread of herpes simplex virus after anterior chamber inoculation. Invest Ophthalmol Vis Sci 32:2462–2472
Whittum JA, McCulley JP, Niederkorn JY, Streilein JW (1984) Ocular disease induced in mice by anterior chamber inoculation of herpes simplex virus. Invest Ophthalmol Vis Sci 25:1065–1073
Yanagi Y, Maekawa R, Cook T, Kanagawa O, Oldstone MBA (1990) Restricted V-segment usage in T cell receptors from cytotoxic T lymphocytes specific for a major epitope of lymphocytic choriomeningitis virus. J Virol 64:5919–5926
Zinkernagel RM, Doherty PC (1979) MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic transplantation antigens determining T-cell restriction-specificity, function and responsiveness. Adv Immunol 27:51–59
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Berra, A., Heiligenhaus, A. & Foster, C.S. T-cell subsets and T-cell receptor Vβ utilization by Igh-1-congenic mice in herpetic retinal necrosis. Graefe's Arch Clin Exp Ophthalmol 234 (Suppl 1), S83–S88 (1996). https://doi.org/10.1007/BF02343053
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02343053