Abstract
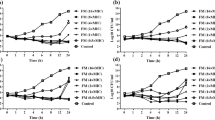
The in vitro initial killing and post-antibiotic effect (PAE) of meropenem on five gramnegative reference strains were evaluated by bioluminescence assay of bacterial adenosine triphosphate (ATP) and viable count. Morphology studies were performed in parallel. Meropenem showed concentration-dependent long (2–5 h) PAEs onEnterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa andSerratia marcescens when assayed by bioluminescence and induced spheroplasts at almost all concentrations. The bioluminescence PAEs reached a maximum response at 4 × MIC. These PAEs of meropenem onEscherichia coli, Klebsiella pneumoniae andSerratia marcescens were longer than corresponding PAEs of imipenem shown in previous studies. The higher affinity of meropenem than imipenem for PBP 3 might explain the longer PAEs obtained with meropenem. However, there was only a very short PAE, no PAE or even a negative PAE when viable count was used as the initial value for the PAE calculation. A strong initial decrease in viability but a less pronounced change in intracellular ATP was registered. Since this initial change in cell numbers is the initial value for the PAE calculation, the length of PAE was highly method dependent. In summary, a strong initial killing and no PAE were shown using viable count as the initial value for the PAE calculation, but a weak initial killing and long PAEs were shown when bioluminescence was used throughout the experiments.
Similar content being viewed by others
References
Edwards JR, Turner PJ, Wannop C, Withnell ES, Grindley AJ, Nairn K: In vitro antibacterial activity of SM-7338, a carbapenem antibiotic with stability to dehydropeptidase I. Antimicrobial Agents and Chemotherapy 1989, 33: 215–222.
Edwards JR, Williams S, Nairn K: Therapeutic activity of meropenem in experimental infections. Journal of Antimicrobial Chemotherapy 1989, 24, Supplement A: 279–286.
Jones RN, Aldridge KE, Allen SD, Barry AL, Fuchs PC, Gerlack EH, Pfaller MA: Multicenter in-vitro evaluation of SM-7338, a new carbapenem. Antimicrobial Agents and Chemotherapy 1989, 33: 562–565.
Jones RN, Barry AL, Thornsberry C: In vitro studies of meropenem. Journal of Antimicrobial Chemotherapy 1989, 24, Supplement A: 9–29.
Moellering RC, Eliopoulos GM, Sentochnik DE: The carbapenems: new broad spectrum β-lactam antibiotics. Journal of Antimicrobial Chemotherapy 1989, 24, Supplement A: 1–7.
Nadler HL, Pitkin DH, Sheikh W: The post-antibiotic effect of meropenem and imipenem on selected bacteria. Journal of Antimicrobial Chemotherapy 1989, 24, Supplement A: 225–231.
Neu HC, Novelli A, Chin NX: In vitro activity and β-lactamase stability of a new carbapenem, SM-7338. Antimicrobial Agents and Chemotherapy 1989, 33: 1009–1018.
Majcherczyk PA, Livermore DM: Penicillin-binding protein (PBP) 2 and the post-antibiotic effect of carbapenems. Journal of Antimicrobial Chemotherapy 1990, 26: 593–594.
Nadler HL, Sheikh W: A comparison of the in vitro postantibiotic effect of meropenem and imipenem versus selectedEnterobacteriaceae and other pathogens. Diagnostic Microbiology and Infectious Diseases 1993, 17: 71–73.
Bundtzen RW, Gerber AU, Cohn DL, Craig WA: Postantibiotic suppression of bacterial growth. Reviews of Infectious Diseases, 1981, 3: 28–37.
Baquero F, Culebras E, Patrón C, Pérez-Díaz JC, Medrano JC, Vicente MF: Postantibiotic effects of imipenem on gram-positive and gram-negative microorganisms. Journal of Antimicrobial Chemotherapy 1986, 18, Supplement E: 47–59.
Isaksson B, Nilsson L, Maller R, Sören L: Postantibiotic effect of aminoglycosides on gram-negative bacteria evaluated by a new method. Journal of Antimicrobial Chemotherapy 1988, 22: 23–33.
Odenholt I, Isaksson B, Nilsson L, Cars O: Postantibiotic and bactericidal effect of imipenem againstPseudomonas aeruginosa. European Journal of Clinical Microbiology & Infectious Diseases 1989, 8: 136–141.
Rennerberg J, Walder M: Postantibiotic effects of imipenem, norfloxacin, and amikacin in vitro and in vivo. Antimicrobial Agents and Chemotherapy 1989, 33: 1714–1720.
Odenholt-Törnqvist I: Pharmacodynamics of beta-lactam antibiotics: studies on the paradoxical and postantibiotic effects in vitro and in animal model. Scandinavian Journal of Infectious Diseases 1989, Supplement 58: 1–55.
Hanberger H, Nilsson LE, Kihlström E, Maller R: Postantibiotic effect of beta-lactam antibiotics onEscherichia coli evaluated by bioluminescence assay of bacterial ATP. Antimicrobial Agents and Chemotherapy 1990, 34: 102–106.
Hanberger H, Nilsson LE, Nilsson M, Maller R: Postantibiotic effect of beta-lactam antibiotics on gram-negative bacteria in relation to morphology, initial killing and MIC. European Journal of Clinical Microbiology & Infectious Diseases 1991, 10: 927–934.
Craig WA, Gudmundsson S: The postantibiotic effect. In: Lorian V (ed): Antibiotics in laboratory medicine. Williams & Wilkins, Baltimore, 1991, p. 403–431.
Hanberger H, Nilsson LE, Svensson E, Maller R: Synergic post-antibiotic effect of mecillinam, in combination with other β-lactam antibiotics in relation to morphology and initial killing. Journal of Antimicrobial Chemotherapy 1991, 28: 523–532.
Hanberger H: Pharmacodynamic effects of antibiotics: studies on bacterial morphology, initial killing, postantibiotic effect and regrowth time. Scandinavian Journal of Infectious Diseases 1992, Supplement 81: 1–52.
Erlandsdottir H, Gudmundsson S: The post-antibiotic effect of imipenem and penicillin-binding protein 2. Journal of Antimicrobial Chemotherapy 1992, 30: 231–232.
Hanberger H, Svensson E, Nilsson M, Nilsson LE, Hörnsten EG, Maller R: Effects of imipenem onEscherichia coli studied using bioluminescence, viable counting and microscopy. Journal of Antimicrobial Chemotherapy 1993, 31: 245–260.
McGrath BJ, Marchbanks CR, Gilbert D, Dudley MN: In vitro postantibiotic effect following repeated exposure to imipenem, temafloxacin and tobramycin. Antimicrobial Agents and Chemotherapy 1993, 37: 1723–1725.
Gutmann L, Vincent S, Billot-Klein D, Acar JF, Mrena E, Williamson R: Involvement of penicillin-binding protein 2 with other penicillin-binding proteins in lysis ofEscherichia coli by some β-lactam antibiotics alone and in synergistic lytic effect of amdinocillin (mecillinam). Antimicrobial Agents and Chemotherapy 1986, 30: 906–912.
Hessen MT, Pitsakis PG, Levison ME: Absence of a postantibiotic effect in experimental pseudomonas endocarditis treated with imipenem, with or without gentamicin. Journal of Infectious Diseases 1988, 158: 542–548.
Wu PJ, Livermore DM: Response of chemostat cultures ofPseudomonas aeruginosa to carbapenems and other β-lactams. Journal of Antimicrobial Chemotherapy 1990, 25: 891–902.
Odenholt-Tornqvist I: Studies on the postantibiotic effect and the postantibiotic sub-MIC effect of meropenem. Journal of Antimicrobial Chemotherapy 1993, 31: 881–892.
Tanio T, Fukasawa M: In vitro and in vivo postantibiotic effect of meropenem. Chemotherapy 1991, 40, Supplement 1: 103–107.
Chapelle EW, Levin GV: Use of firefly, bioluminescent reaction for rapid detection and counting of bacteria. Biochemical Medicine 1968, 2: 41–52.
Nilsson L: New rapid bioassay of gentamicin based on luciferase assay of extracellular ATP in bacterial cultures. Antimicrobial Agents and Chemotherapy 1978, 14: 812–816.
Nilsson L: Luciferase assay of bacterial ATP as a tool for rapid antibiotic assay. Medical Dissertation No. 109, Linköping University, Linköping, Sweden, 1981.
Thore A, Lundin A, Bergman S: Detection of bacteriuria by luciferase assay of adenosine triphosphate. Journal of Clinical Microbiology 1975, 1: 1–8.
Molin Ö, Nilsson L, Ånsehn S: Rapid detection of bacterial growth in blood cultures by bioluminescent assay of bacterial ATP. Journal of Clinical Microbiology 1983, 18: 521–525.
Kronvall G, Myhre E: Differential staining of bacteria in clinical specimens using acridine orange buffered at a low pH. Acta Pathologica et Microbiologica Scandinavica (B) 1977, 58: 249–254.
Kitzis MD, Acar JF, Gutmann L: Antibacterial activity of meropenem against gram-negative bacteria with a permeability defect and against staphylococci. Journal of Antimicrobial Chemotherapy 1989, 24, Supplement A: 125–132.
Sumita Y, Fukasawa M, Okuda T: Comparison of two carbapenems, SM-7338 and imipenem: affinities for penicillin-binding proteins and its release from these proteins and morphological changes. Journal of Antibiotics 1990, 42: 314–320.
Hayes MV, Orr DC: Mode of action of ceftazidime: affinity for the penicillin binding proteins ofEscherichia coli K 12,Pseudomonas aeruginosa andStaphylococcus aureus. Journal of Antimicrobial Chemotherapy 1983, 12: 119–126.
Neu HC: Penicillin-binding proteins and beta-lactamases: their effects on the use of cephalosporins and other beta-lactams. In: Remington J, Swartz MN (ed): Current clinical topics in infectious diseases. McGraw-Hill, New York, 1987, p. 37–47.
Odenholt-Tornqvist I, Löwdin E, Cars O: Pharmacodynamic effects of subinhibitory concentrations of β-lactam antibiotics in vitro. Antimicrobial Agents and Chemotherapy 1991, 35: 1834–1839.
Oshida T, Onta T, Nakanishi N, Matsushita T, Yamaguchi T: Activity of subminimal inhibitory concentrations of aspoxicillin in prolonging the postantibiotic effect againstStaphylococcus aureus. Journal of Antimicrobial Chemotherapy 1990, 26: 29–38.
Odenholt I, Holm SE, Cars O: Effects of supra-MIC and sub-MIC benzylpenicillin concentrations on group A β-hemolytic streptococci during the postantibiotic phase in vivo. Journal of Antimicrobial Chemotherapy 1990, 26: 193–201.
Craig WA, Ebert SC: Continuous infusion of β-lactam antibiotics. Antimicrobial Agents and Chemotherapy 1992, 12: 2577–2583.
Cars O, Craig WA: General discussion on antibiotic dosing. Scandinavian Journal of Infectious Diseases 1990, Supplement 74: 282–283.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hanberger, H., Svensson, E., Nilsson, L.E. et al. Pharmacodynamic effects of meropenem on gram-negative bacteria. Eur. J. Clin. Microbiol. Infect. Dis. 14, 383–390 (1995). https://doi.org/10.1007/BF02114893
Issue Date:
DOI: https://doi.org/10.1007/BF02114893