Summary
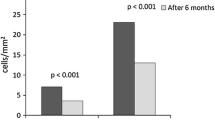
We examined the relationship between interstitial cell infiltration and myocardial fibrosis in patients with clinically diagnosed dilated cardiomyopathy (DCM). Forty-two cases of DCM were divided into two groups, according to the mean number of the interstitial round cells per 10.2×104 square µm (Nic): the inflammatory group (Nic ≥5) and noninflammatory group (Nic<5). The 12 cases in the inflammatory group were clinically similar to the 30 cases in the non-inflammatory group, but the inflammatory group exhibited a significantly (P<0.001) larger area of myocardial fibrosis (34.8%±12.8% vs 17.5%±8.2%), a significantly (P<0.01) higher frequency of diffuse perimyocytic-type fibrosis (83% vs 23%), fewer myocardial cells in the left ventricular wall (170±70 fibers vs 216±81 fibers), and significantly (P<0.01) greater hypertrophy of the myocytes (18.3±3.4 vs 15.3±2.7 µm). In addition, cases exhibiting marked fibrosis (fibrosis area ≥25% of the myocardium) had a significantly higher Nic score (8.3±6.8) compared to cases with the less fibrotic type of DCM (4.0±5.7).
We speculate that persistent or preceding inflammatory cell infiltration induces the myocardial fibrosis, especially the diffuse perimyocytic type, in the fibrosis-predominant type of DCM. Therefore, most of these cases may be a sequela of myocarditis, and more correctly termed post-myocarditic cardiomegaly.
Similar content being viewed by others
References
WHO/ISFC (1980) Report of the WHO/ISFC task force on the definition and classification of cardiomyopathies. Br Heart J 40: 63–65
Goodwin JF (1982) The frontiers of cardiomyopathies. Heart J 48: 1–18
Kawai S, Okada R (1987) A histopathological study of dilated cardiomyopathy—with special reference to clinical and pathological comparisons of the degeneration-predominant type and fibrosis-predominant type. Jpn Circ J 51: 654–660
Kunkel B, Lapp H, Kaltenbach M (1978) Correlations between clinical and morphologic findings and natural history in congestive cardiomyopathy. In: Kaltenbach M, Loogen F, Olsen EGJ (eds) Cardiomyopathy and myocardial biopsy. Springer, Berlin, pp 271–283
Baandrup U, Florio RA, Olsen EGJ (1982) Do endomyocardial biopsies represent the morphology of the rest of the myocardium? A quantitative light microscopic study of single v. multiple biopsies with King's bioptome. Eur Heart J 3: 171–178
Edwards WD, Holms DR Jr, Reeder GS (1982) Diagnosis of active lymphocytic myocarditis by endomyocardial biopsy: Quantitative criteria for light microscopy. Mayo Clin Proc 57: 419–425
Nippoldt TB, Edwards WD, Holmes DR, Reeder GS, Hartlzer GO, Smith HC (1982) Right ventricular endomyocardial biopsy. Clinicopathologic correlates in 100 consecutive patients. Mayo Clin Proc 57: 407–418
Fenoglio JJ Jr, Ursell PC, Kellogg CF, Drusin RE, Weiss MB (1983) Diagnosis and classification of myocardial biopsy. N Engl J Med 308: 12–18
Parrillo JE, Aretz HT, Palacios I, Fallon JT, Block PC (1984) The results of transvenous endomyocardial biopsy can frequently be used to diagnose myocardial diseases in patients with idiopathic heart failure. Endomyocardial biopsies in 100 consecutive patients revealed a substantial incidence of myocarditis. Circulation 69: 93–101
Zee-Cheng C, Tsai CC, Palmer DC, Codd JE, Pennington DG, Williams GA (1984) High incidence of myocarditis by endomyocardial biopsy in patients with idiopathic congestive cardiomyopathy. J Am Coll Cardiol 3: 363–70
Dec GW Jr, Palacios IF, Fallon JT, Aretz HT, Mills J, Lee DCS, Johnson RA (1985) Active myocarditis in the spectrum of acute dilated cardiomyopathies. Clinical features, histologic correlates, and clinical outcome. N Engl J Med 312: 885–890
Cassling RS, Linder J, Sears TD, Waller BF, Rogler WC, Wilson JE, Kugler JD, Kay DH, Dillon JC, Slack JD, McManus BM (1985) Quantitative evaluation of inflammation in biopsy specimens from idiopathically failing or irritable hearts: Experience in 80 pediatric and adult patients. Am Heart J 110: 713–720
French WJ, Siegel RJ, Cohen AH, Laks MM (1986) Yield of endomyocardial biopsy in patients with biventricular failure. Comparison of patients with normal vs reduced left ventricular ejection fraction. Chest 90: 181–184
Latham RD, Murlow JP, Virmani R, Robinowitz M, Moody JM (1989) Recently diagnosed idiopathic dilated cardiomyopathy: Incidence of myocarditis and efficacy of prednisone therapy. Am Heart J 117: 876–882
Billingham M (1987) Acute myocarditis: a diagnostic dilemma. Br Heart J 58: 6–8
Aretz HT, Billingham ME, Edwards WD, Factor SM, Fallon JT, Fenoglio JJ Jr, Olsen EGJ, Schoen FJ (1986) Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol 1: 3–14
Aretz HT (1987) Myocarditis: The Dallas criteria. Hum Pathol 18: 619–624
Kline IK, Saphir O (1960) Chronic pernicious myocarditis. Am Heart J 59: 681–697
Hirshman S, Hammer GS (1974) Coxsackie Virus Myopericarditis. A microbiological and clinical review. Am J Cardiol 34: 224–232
Lenghaus C, Studdert M (1984) Acute and chronic viral myocarditis. Acute diffuse nonsuppurative myocarditis and residual myocardial scarring following infection with canine parvovirus. Am J Pathol 115: 316–319
O'Connell JB, Robinson JA, Gunnar RM, Scanlon PJ (1985) Clinical aspects of virus/immune myocarditis. In: Sekiguchi M, Olsen EGJ, Goodwin JF (eds) Myocarditis and related disorders. Springer-Verlag, Tokyo Berlin Heidelberg New York, pp 102–106
Levi G, Scalvini S, Volterrani M, Marangoni S, Arosio G, Quadri A (1988) Coxsackie virus heart disease: 15 years after. Eur Heart J 9: 1303–1307
Postlethwaite AE, Synderman R, Kang AH (1976) The chemotactic attraction of human fibroblasts to a lymphocyte-derived factor. J Exp Med 144: 1188–1203
Tsukamoto Y, Helsel WE, Wahl SM (1982) Macrophage production of fibronectin, a chemoattractant for fibroblasts. J Immunol 127: 673–678
Huber SA, Jodge PA, Job LP (1984) The role of virus-and immune-mediated cardiocyte injury in Coxsackievirus B3-induced myocarditis. In: Bolte H-D (ed) Viral heart disease. Springer-Verlag, Berlin Heidelberg New York Tokyo, pp 64–73
Bendinelli M, Matteucci D, Conaldi PG, Giangregorio AM, Capobianchi MR, Dianzani F (1987) Mechanisms of group B coxsackie virus persistence in human cells. Eur Heart J 8 (Suppl. J): 441–444
Kandolf R, Hofschneider PH (1984) Effect of interferon on the replication of Coxsackie B3 virus in cultured human fetal heart cells. In: Bolte H-D (ed) Viral heart disease. Springer-Verlag, Berlin Heidelberg New York Tokyo, pp 57–63
Bowles NE, Richardson PJ, Olsen EGJ, Archard LC (1986) Detection of Coxsackie-B-virus-specific RNA sequences in myocardial biopsy samples from patients with myocarditis and dilated cardiomyopathy. Lancet II: 1120–1122
Maish B (1985) Immunologic regulator and effector mechanisms in myocarditis and pericarditis. In: Sekiguchi M, Olsen EGJ, Goodwin JF (eds) Myocarditis and related disorders. Springer-Verlag, Tokyo Berlin Heidelberg New York, pp 209–217
Huber SA, Lodge PA, Herzum M, Estrin M, Olszewski J (1987) The role of T lymphocytes in the pathogenesis of Coxsackievirus B3 myocarditis. In: Kawai C, Abelmann WH (eds) Pathogenesis of myocarditis and cardiomyopathy. Recent experiments and clinical studies. University of Tokyo Press, Tokyo, pp 9–21
Maisch B (1984) Cardiocytolysis by sera of patients suffering from acute myocarditis. In: Bolte H-D (ed) Viral heart disease. Springer-Verlag, Berlin Heidelberg New York Tokyo, pp 121–130
Dinarello CA (1987) Lymphokines. N Engl J Med 317: 940–945
Johnson RF, Ziff M (1976) Lymphokine stimulation of collagen accumulation. J Clin Invest 58: 240–252
Schmidt JA, Mizel SB, Cohen D, Green I (1982) Interleukin 1. A potent regulator of fibroblast proliferation. J Immunol 128: 2177–2188
Agelli M, Wahl SM (1986) Cytokines and fibrosis. Clin Exp Rheumatol 4: 379–388
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kawai, S., Okada, R. Interstitial cell infiltrate and myocardial fibrosis in dilated cardiomyopathy: A special type of cardiomegaly corresponding to sequelae of myocarditis. Heart Vessels 5, 230–236 (1990). https://doi.org/10.1007/BF02058695
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02058695