Summary
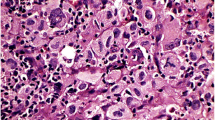
The occurrence and the distribution of cytokeratin (CK)-immunoreactive reticulum cells in a series of normal and pathological human lymph nodes and spleens are documented. The immunoreactive cells exhibit morphological and immunophenotypic features of so-called fibroblastic reticulum cells, with or without myoid differentiation. They invariably co-express vimentin and, to a lesser extent, desmin and muscle-specific actin isoforms. These CK-immunoreactive cells are apparently a normal subpopulation of reticulum cells, being detectable from the early stages of spleen and lymph node development. They are distributed mainly in the paracortical and medullary regions of the lymph nodes and at the periphery of the white pulp in the spleen. Their number and distribution are highly variable in different neoplastic and non-neoplastic pathological conditions but the changes are not disease specific. CK-immunoreactive reticulum cells are easily identifiable in both frozen and fixed lymphoid tissue and in cytological smears of fine-needle aspirates, provided that monoclonal antibodies whose spectrum of reactivity includes cytokeratins 8 and 18 are used. The awareness of the occurrence of CK-immunoreactive cells in normal lymphoid tissues is of particular relevance in the search for micrometastatic foci using anti-CK antibodies.
Similar content being viewed by others
References
Angus B, Purvis J, Stock D, Westley BR, Samson ACR, Routledge EG, Carpenter FH, Horne CHW (1987) NCL-5D3: a new monoclonal antibody recognizing low molecular weight cytokeratins effective for immunocytochemistry using fixed paraffin-embedded tissue. J Pathol 153:377–384
Battifora H (1987) Diagnostic uses of antibodies to keratins. A review and immunohistochemical comparison of seven monoclonal and three polyclonal antibodies. In: Fenoglio C, Wolff M, Rilke F (eds) Progress in surgical pathology. Masson, New York, pp 1–15
Battifora H (1988a) The biology of the keratins and their diagnostic applications. In: De Lellis RA (ed) Advances in immunohistochemistry. Raven Press, New York, pp 191–221
Battifora H (1988b) Clinical applications of the immunohistochemistry of filamentous proteins. Am J Surg Pathol 12:24–42
Broers JLV, Carney DN, Rot MK, Schaart G, Lane EB, Vooijs GP, Ramaekers FCS (1986) Intermediate filament proteins in classic and variant types of small cell lung carcinoma cell lines: a biochemical and immunochemical analysis using a panel of monoclonal and polyclonal antibodies. J Cell Sci 83:37–60
Cattoretti G, Berti E, Schiro' R, D'Amato L, Valeggio C, Rilke F (1988) Improved avidin-biotin-peroxidase complex (ABC) staining. Histochem J 20:75–80
Chan R, Edwards B, Hu R, Rossitto PV, Min BH, Lund JK, Cardiff RD (1988) Characterization of two monoclonal antibodies in an immunohistochemical study of keratin 8 and 18 expression. Am J Clin Pathol 89:472–480
Cooper D, Schermer A, Sun TT (1985) Classification of human epithelia and their neoplasms using monoclonal antibodies to keratins: strategies, applications and limitations. Lab Invest 52:243–256
Cordell JL, Falini B, Erber WN, Ghosh AK, Abdulaziz Z, MacDonald S, Pulford KAF, Stein H, Mason DY (1984) Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem 32:219–229
Czernobilsky B, Moll R, Levy R, Franke WW (1985) Co-expression of cytokeratin and vimentin filaments in mesothelial, granulosa and rete ovarii cells of the human ovary. Eur J Cell Biol 37:175–190
Debus E, Weber K, Osborn M (1983) Monoclonal antibodies to desmin, the muscle-specific intermediate filaments protein. EMBO J 2:2305–2312
Debus E, Moll R, Franke WW, Weber K, Osborn M (1984) Immunohistochemical distinction of human carcinomas by cytokeratin typing with monoclonal antibodies. Am J Pathol 114:121–130
Delsol G, Al Saati T, Gatter KC, Gerdes J, Schwarting R, Caveriviere P, Rigal-Huguet F, Robert A, Stein H, Mason DY (1988) Coexpression of epithelial membrane antigen (EMA), Ki-1, and interleukin-2 receptor by anaplastic large cell lymphomas. Diagnostic value in so-called malignant histiocytosis. Am J Pathol 130:59–70
De Mascarel A, Merlio J-P, Coindre J-M, Goussot J-F, Broustet A (1989) Gastric large cell lymphoma expressing cytokeratin but no leukocyte common antigen. A diagnostic dilemma. Am J Clin Pathol 91:478–481
Doglioni C, Dell'Orto P, Coggi G, Iuzzolino P, Bontempini L, Viale G (1987) Choroid plexus tumors. An immunocytochemical study with particular reference to the coexpression of intermediate filament proteins. Am J Pathol 127:519–529
Franke WW, Moll R (1987) Cytoskeletal components of lymphoid organs. I. Synthesis of cytokeratins 8 and 18 and desmin in subpopulations of extrafollicular reticulum cells of human lymph nodes, tonsils and spleen. Differentiation 36:145–163
Franke WW, Winter S, Overbeck J von, Gudat F, Heitz PU, Stähli C (1987) Identification of the conserved, conformation-dependent cytokeratin epitope recognized by monoclonal antibody (lu-5). Virchows Arch [A] 411:137–147
Gabbiani G, Kapanci Y, Barazzone P, Franke WW (1981) Immunochemical identification of intermediate-sized filaments in human neoplastic cells. Am J Pathol 104:206–216
Gould VE, Shin SS, Manderino GL, Rittenhouse HG, Tomita JT, Gouch GT (1988) Selective expression of a novel mucin-type glycoprotein in human tumors. Immunohistochemical demonstration with Mab A-80. Hum Pathol 19:623–627
Gown AM, Vogel AM (1984) Monoclonal antibodies to human intermediate filament proteins. II. Distribution of filament proteins in normal tissues. Am J Pathol 114:309–321
Gown AM, Boyd HC, Chang Y, Ferguson M, Reichler B, Tippens D (1988) Smooth muscle cells can express cytokeratins of “simple” epithelium. Immunocytochemical and biochemical studiesin vitro andin vivo. Am J Pathol 132:223–232
Holthöfer H, Miettinen A, Lehto V-P, Linder E, Alfthen O, Virtanen I (1983) Cellular origin and differentiation of renal cell carcinomas. A fluorescence microscopic study with kidney-specific antibodies, antiintermediate filament antibodies and lectins. Lab Invest 50:552–559
Hsu S-M, Raine L, Fanger H (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques. A comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29:577–580
Iuzzolino P, Bontempini L, Doglioni C, Zanetti G (1989) Keratin immunoreactivity in extrafollicular reticular cells of the lymph node (letter). Am J Clin Pathol 91:239–240
Jack AS, Grigor I, O'Brien CJ, McMeekin W, Lewis F, McNicol AM (1989) Association between CAM 5.2 and anti-CD1a reactivity in lymph nodes and gastrointestinal tract epithelium. J Clin Pathol 42:271–274
Jahn L, Fouquet B, Rohe K, Franke WW (1987) Cytokeratins in certain endothelial and smooth muscle cells of two taxonomically distinct vertebrate species,Xenopus laevis and man. Differentiation 36:234–254
Makin CA, Bobrow LG, Bodmer WF (1984) Monoclonal antibody to cytokeratin for use in routine histopathology. J Clin Pathol 37:975–983
Miettinen M, Virtanen I, Talerman A (1985) Intermediate filament proteins in human testis and testicular germ-cell tumors. Am J Pathol 120:402–410
Mujien GNP van, Ruiter DJ, Warnaar SO (1987) Coexpression of intermediate filament polypeptides in human fetal and adult tissues. Lab Invest 57:359–369
Nagle RB, Bocker W, Davis JR, Heid HW, Kaufmann M, Lucas DO, Jarash E-D (1986) Characterization of breast carcinomas by two monoclonal antibodies distinguishing myoepithelial from luminal epithelial cells. J Histochem Cytochem 34:869–881
Naiem M, Gerdes J, Abdulaziz Z, Stein H, Mason DY (1983) Production of a monoclonal antibody reactive with human dendritic reticulum cells and its use in the immunohistological analysis of lymphoid tissue. J Clin Pathol 36:167–175
Osborn M, Weber K (1983) Tumor diagnosis by intermediate filament typing: a novel tool for surgical pathology. Lab Invest 48:372–394
Osborn M, Debus E, Weber K (1984) Monoclonal antibody specific for vimentin. Eur J Cell Biol 34:137–143
Overbeck J von, Stähli C, Gudat F, Carmann H, Lautenshlager C, Durmuller U, Takacs B, Miggiano V, Staehelin T, Heitz PU (1985) Immunohistochemical characterization of an antiepithelial monoclonal antibody (mAb lu-5). Virchows Arch [A] 407:1–12
Pinkus GS, Warhol MJ, O'Connor EM, Etheridge CL, Fujiwara K (1986) Immunohistochemical localization of smooth muscle myosin in human spleen, lymph node, and other lymphoid tissues: unique staining patterns in splenic white pulp and sinuses, lymphoid follicles, and certain vasculature, with ultrastructural correlations. Am J Pathol 123:440–453
Ramaekers FCS, Vooijs GP, Huijsmans ACLM, Salet-v.d. Pol MRJ, van Aspert-van Erp AJM, Beck HLM (1988) Immunocytochemistry as an aid in diagnostic cytopathology. In: De Lellis RA (ed) Advances in immunohistochemistry. Raven Press, New York, pp 133–163
Sewell HF, Thompson WD, King DJ (1986) IgD myeloma/immunoblastic lymphoma cells expressing cytokeratin. Br J Cancer 53:695–696
Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G (1986) A monoclonal antibody against α-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol 103:2787–2796
Stansfeld AG, Diebold J, Kapanci Y, Kelenyi G, Lennert K, Mioduszewska O, Noel H, Rilke F, Sundstrom C, Unnik JAM van, Wright DH (1988) Updated Kiel classification for lymphomas. Lancet I:292–293
Toccanier-Pelte M-F, Skalli O, Kapanci Y, Gabbiani G (1987) Characterization of stromal cells with myoid features in lymph nodes and spleen in normal and pathologic conditions. Am J Pathol 129:109–118
Tsukuda T, Tippens D, Gordon D, Ross R, Gown AM (1987) HHF 35, a muscle-actin-specific monoclonal antibody. Am J Pathol 126:51–60
Turley H, Pulford KAF, Gatter KC, Mason DY (1988) Biochemical evidence that cytokeratins are present in smooth muscle. Br J Exp Pathol 69:433–440
Tykocinski M, Schinella RA, Greco A (1983) Fibroblastic reticulum cells in human lymph nodes. An ultrastructural study. Arch Pathol Lab Med 107:418–422
Viac J, Reano A, Brochier J, Staquet M-J, Thivolet J (1983) Reactivity pattern of a monoclonal antikeratin antibody (KL1). J Invest Dermatol 81:351–354
Viale G (1989) Cytokeratin immunocytochemistry in the practice of diagnostic histopathology (letter). Ultrastruct Pathol 13:91
Wotherspoon AC, Norton AJ, Isaacson PG (1989) Immunoreactive cytokeratins in plasmacytomas. Histopathology 14:141–150
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Doglioni, C., Dell'Orto, P., Zanetti, G. et al. Cytokeratin-immunoreactive cells of human lymph nodes and spleen in normal and pathological conditions. Vichows Archiv A Pathol Anat 416, 479–490 (1990). https://doi.org/10.1007/BF01600298
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01600298