Summary
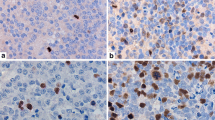
Cell kinetic study plays an important role in treatment planning of brain tumour patients. MIB-1 antibody has recently become available, which detects Ki-67 antigen even in the formalin-fixed paraffin-embedded specimens. We performed MIB-1 immunostaining in 50 meningiomas and 50 neurinomas, and estimated the cell cycle time (tc) and potential doubling time (Tpot) from MIB-1 staining index (MIB-1 SI) and mitotic index (MI). MIB-1 SI logarithmically correlated with MI in both meningiomas and neurinomas. The tc and the Tpot were expressed as a function of the mitosis time (tm), while the tm is known to be around one hour and not exceeding two hours. When the tm was assumed to be one hour, the average tcs of meningiomas and neurinomas were 6.53±3.56 days and 7.67±3.27 days, respectively. The Tpots were447 × (MIB-1 SI)−1.29 × tm in meningiomas, and490 × (MIB-1 SI) −0.98 × tm in neurinomas.
The tumour doubling times (Tds) were calculated from serial imaging studies in 22 neurinomas and 15 meningiomas. The Tds were formulated as794 × (MIB-1 SI) −0.83 in meningiomas and1380 × (MIB-1 SI) −0.97 in neurinomas. Most of the Tds correlated well with the Tpots in meningiomas and neurinomas, and exceeded values of the Tpot when the tm is assumed to be one hour, although a few tumours showed unexpectedly longer Tds. The Tpot and the tc estimated from MIB-1 SI and MI are clinically useful parameters for predicting the growth potential of meningiomas and neurinomas where no other simple methods are available.
Similar content being viewed by others
References
Bederson J, von Ammon K, Wichmann WW, Yasargil MG (1991) Conservative treatment of patients with acoustic tumors. Neurosurgery 28: 646–651
Bruno S, Darzynkiewicz Z (1992) Cell cycle dependent expression and stability of the nuclear protein detected by Ki67 antibody in HL-60 cells. Cell Prolif 25: 31–40
Burger PC, Shibata T, Kleihues P (1986) The use of the monoclonal antibody Ki-67 in the identification of proliferating cells. Application to surgical neuropathology. Am J Surg Pathol 10: 611–617
Cattoretti G, Becker MHG, Key G, Duchrow M, Schlüter C, Galle J, Gerdes J, Schwab U, Lemke H, Stein H (1983) Production of a mouse monoclonal antibody reactive with human nuclear antigen associated with cell proliferation. Int J Cancer 31: 13–20
Cho KG, Hoshino T, Nagashima T, Murovic JA, Wilson CB (1986) Prediction of tumor doubling time in recurrent meningiomas. J Neurosurg 65: 790–794
Clever JE (1967) Thymidine metabolism and cell kinetics. North-Holland, Amsterdam, pp 125–132
Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H (1984) Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 133: 1710–1715
Gerdes J (1992) Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB-1 and MIB-3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol 168: 357–363
Hall PA, Woods AL (1990) Immunohistochemical markers of cellular proliferation: achievements, problems and prospects. Cell Tissue Kinet 23: 505–522
Hoshino T, Wilson CB (1979) Cell kinetic analyses of human malignant brain tumors (gliomas). Cancer 44: 956–962
Hoshino T, Ito S, Asai A, Shibuya M, Prados MD, Dodson BA, Davis RL, Wilson CB (1992) Cell kinetic analysis of human brain tumors by in situ double labeling with bromodeoxyuridine and iododeoxyuridine. Int J Cancer 50: 1–5
Kalamitopoulou E, Peentes E, Diamantis I, Maraziotis T (1994) Ki-67 immunoreactivity in human central nervous system tumors: a study with MIB-1 monoclonal antibody on archival material. Acta Neuropathol 87: 47–54
Kameyama S, Tanaka R, Honda Y, Hasegawa A, Yamazaki H, Kawaguchi T (1994) The long-term growth rate of residual acoustic neurinomas. Acta Neurochir (Wien) 129: 127–130
Kerr KM, Lamb D (1984) Actual growth rate and tumor cell proliferation in human pulmonary neoplasms. Br J Cancer 50: 343–349
Key G, Becker MHG, Baron B, Duchrow M, Schlüter C, Flad H-D, Gerdes J (1993) New Ki-67-equivalent murine monoclonal antibodies (MIB 1–3) generated against bacterially expressed parts of the Ki-67 cDNA containing three 62 base pair repetitive elements encoding for the Ki-67 epitope. Lab Invest 68: 629–636
Landberg G, Tan EM, Roos G (1990) Flow cytometric multiparameter analysis of proliferating cell nuclear antigen/cyclin and Ki-67 antigen: a new view of the cell cycle. Exp Cell Res 187: 111–118
Lesser THJ, Janzer RC, Kleihues P, Fisch U (1991) Clinical growth rate of acoustic schwannomas. Correlation with the growth fraction as defined by the monoclonal antibody Ki-67. Skull Base Surg 1: 11–15
Lopez F, Belloc F, Lacombe F, Dumain P, Reiffers P, Bernard P, Boisseau MR (1994) The labeling of proliferating cells by Ki67 and MIB-1 antibodies depends on the binding of a nuclear protein to the DNA. Exp Cell Res 210: 145–153
Meyer JS (1981) Growth and cell kinetic measurements in human tumors. Pathol Ann 16: 53–80
Moore JV (1987) Death of cells and necrosis of tumors. In: Potten CS (ed) Perspectives in mammalian cell death. Oxford University Press, Oxford, pp 295–325
Morimura T, Kitz K, Budka H (1989) In situ analysis of cell kinetics in human brain tumors. A comparative immunocytochernical study of S phase cells by a new in vitro bromodeoxy-uridine-labeling technique, and of proliferating pool cells by monoclonal antibody Ki-67. Acta Neuropathol 77: 276–282
Parkins CH, Darling JL, Gill SS, Revesz T, Thomas DG (1991) Cell proliferation in serial biopsies through human malignant brain tumours: measurement using Ki-67 antibody labeling. Br J Neurosurg 5: 289–298
Raghavan R, Steart PV, Weller RO (1990) Cell proliferation patterns in the diagnosis of astrocytomas, anaplastic astrocytomas and glioblastoma multiforme: a Ki-67 study. Neuropathol Appl Neurobiol 16: 123–133
Roggendorf W, Schuster T, Pfeiffer J (1987) Proliferative potential of meningiomas determined with the monoclonal antibody Ki-67. Acta Neuropathol 73: 361–364
Santisteaban MS, Brugal G (1994) Image analysis of in situ cell cycle related changes of PCNA and Ki-67 proliferating antigen expression. Cell Prolif 27: 435–453
Schröder R, Bien K, Kott R, Meyers I, Vössing R (1991) The relationship between Ki-67 labeling and mitotic index in gliomas and meningiomas: demonstration of the variability of the intermitotic cycle time. Acta Neuropathol 82: 389–394
Shibuya M, Ito S, Davis R, Wilson CB, Hoshino T (1993) A new method for analyzing the cell kinetics of human brain tumors by double labeling with bromodeoxyuridine in situ and with iododeoxyuridine in vitro. Cancer 71: 3109–3113
Steel GG (1968) Cell loss from experimental tumors. Cell Tissue Kinet 1: 193–207
Van Dierendonck JH, Keijzer R, van de Velde CJH, Cornelisse CJ (1989) Nuclear distribution of the Ki-67 antigen during the cell cycle: comparison with growth fraction in human breast cancer cells. Cancer Res 49: 2999–3006
Woosley JT (1991) Measuring cell proliferation. Arch Pathol Lab Med 115: 555–557
Yoshii Y, Maki Y, Tsuboi K, Tomono Y, Nakagawa K, Hoshino T (1986) Estimation of growth fraction with bromodeoxy uridine in human central nervous system tumors. J Neurosurg 65: 659–663
Yu CC-W, Woods AL, Levison DA (1992) The assessment of cellular proliferation by immunohistochemistry: a review of currently available methods and their applications. Histochem J24: 121–131
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nakasu, S., Nakasu, Y., Nakajima, M. et al. Potential doubling time and tumour doubling time in meningiomas and neurinomas. Acta neurochir 138, 763–770 (1996). https://doi.org/10.1007/BF01411485
Issue Date:
DOI: https://doi.org/10.1007/BF01411485