Summary
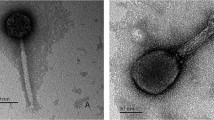
A comparative study is reported on five phages of theMyoviridae family which propagate onBacillus subtilis, B. thuringiensis, Enterococcus sp.,Lactobacillus plantarum, orStaphylococcus aureus. The phages are morphologically identical and characterized by isometric heads with conspicuous capsomers and by contractile tails with complex base plates. The phages show similar protein profiles, but vary considerably in burst size. Phage DNAs are about 95–166kb in size and are unrelated by DNA-DNA hybridization and restriction endonuclease analysis. Therefore the phages are unrelated at species level. Implications of these data for our understanding of the development of phage species are discussed.
Similar content being viewed by others
References
Ackermann H-W, DuBow MS (1987 a) Viruses of prokaryotes. I. General properties of bacteriophages. CRC Press, Boca Raton, pp 179–182
Ackerman H-W, DuBow MS (1987 b) Viruses of prokaryotes. II. Natural groups of bacteriophages. CRC Press, Boca Raton, pp 51–161
Ackermann H-W, DuBow MS, Jarvis AW, Jones LA, Krylov VN, Maniloff J, Rocourt J, Safferman RS, Schneider J, Seldin L, Sozzi T, Stewart PR, Werquin M, Wünsche L (1992) The species concept and its application to tailed phages. Arch Virol 124: 69–82
Ackermann H-W, Greer GG, Rocourt J (1988) Morphology ofBrochothrix thermosphacta phages. Microbios 56: 19–26
Braun V, Hertwig S, Neve H, Geis A, Teuber M (1989) Taxonomic differentiation of bacteriophages ofLactococcus lactis by electron microscopy, DNA-DNA hybridization and protein profiles. J Gen Microbiol 135: 2551–2560
Francki RIB, Fauquet CM, Knudson DL, Brown F (eds) (1991) Classification and nomenclature of viruses. Fifth Report of the International Committee on Taxonomy of Viruses. Springer, Wien New York (Arch Virol [Suppl]2)
Jarvis AW (1978) Serological studies of a host range mutant of a lactic streptococcal bacteriophage. Appl Environ Microbiol 36: 785–789
Jarvis AW, Fitzgerald GF, Mata M, Mercenier A, Neve H, Powell IB, Ronda C, Saxelin M, Teuber M (1991) Species and type phages of lactococcal bacteriophages. Intervirology 32: 2–9
Jarvis AW, Meyer J (1986) Electron microscopic heteroduplex study and restriction endonuclease analysis of the DNA genomes of three lactococcal bacteriophages. Appl Environ Microbiol 51: 1272–1277
Klaenhammer TR, Sanozky RB (1985) Conjugal transfer fromStreptococcus lactis ME 2 of plasmids encoding phage resistance, nisin resistance and lactose-fermenting ability: evidence for a high-frequency conjugative plasmid responsible for abortive infection of virulent bacteriophage. J Gen Microbiol 131: 1531–1541
Korsten KH, Tomkiewicz T, Hausmann R (1979) The strategy of infection as a criterion for phylogenetic relationships of non-coli phage morphologically similar to phage T7. J Gen Microbiol 43: 57–73
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685
London J, Kline K (1973) Aldolase of lactic acid bacteria: a case history in the use of an enzyme as evolutionary marker. Bacteriol Rev 37: 453–478
Ludwig W, Schleifer K-H, Stackebrandt E (1984) 16 S rRNA analysis ofListeria monocytogenes andBrochothrix thermosphacta. FEMS Microbiol Lett 25: 199–204
Luftwig R (1976) An accurate measurement of the catalase crystal period and its use as an internal marker for electron microscopy. J Ultrastruct Res 20: 91–102
Southern EM (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98: 503–517
Tikhonenko AS (1970) Ultrastructure of bacterial viruses. Plenum Press, New York, pp 143–145
Twort FW (1915) An investigation on the nature of ultramicroscopic viruses. Lancet 2: 1241
Ward LJH, Jarvis AW (1992) Rapid removal of cesium chloride from DNA obtained from ultracentrifuge gradients. Biotechnics 12: 12
Woese CR (1987) Bacterial evolution. Microbiol Rev 51: 221–271
Zink R, Loessner MJ (1992) Classification of virulent and temperate bacteriophages ofListeria spp. on the basis of morphology and protein analysis. Appl Environ Microbiol 58: 296–302
Author information
Authors and Affiliations
Additional information
Chairperson
Vice Chairperson of ICTV Bacterial Virus Subcommittee
Rights and permissions
About this article
Cite this article
Jarvis, A.W., Collins, L.J. & Ackermann, H.W. A study of five bacteriophages of theMyoviridae family which replicate on different gram-positive bacteria. Archives of Virology 133, 75–84 (1993). https://doi.org/10.1007/BF01309745
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01309745