Abstract
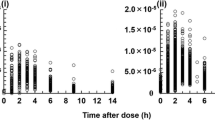
To account for the individual variability in cyclosporine pharmacokinetics and the non-existence of dosing recommendations in young children, we studied the pharmacokinetics of cyclosporine before renal transplantation in ten children aged 1.1–2.5 years, to determine the appropriate individual dose. Our aim was to reach a steady-state cyclosporine blood level of 200–300 μg/l, 8 h after a dose in the first days after renal transplantation. Cyclosporine was given as a single oral dose (10 mg/kg) or as a 4-h i. v. infusion (3 mg/kg), and the blood concentration was determined for 24 h by a specific monoclonal radioimmunoassay. The mean terminal cyclosporine half-life (t 1/2) was 9.3 h (range 2.8–20.4), blood clearance 10.8 ml/min per kilogram (range 6.8–22.7) and volume of distribution 2.8 l/kg (range 1.4–4.7). The bioavailability of oral cyclosporine was low; the mean amount absorbed was 21.8% of the administered dose (range 11–35). The mean calculated dose needed to attain the intended predose blood cyclosporine level of 200–300 μg/l at steady-state was 5 mg/kg per day for i.v. and 21 mg/kg per day for oral administration. In view of the shortt 1/2, we used three doses/day. The validity of the predicted doses is shown by the mean cyclosporine doses used during the first 10 days after transplantation, which were 93.5% of the calculated oral and 96.6% of the calculated i.v. doses. The observed mean cyclosporine concentration during the same period was 196 μg/l.
Similar content being viewed by others
References
Yee GC, Lennon TP, Gmur DJ, Kennedy MS, Deeg HJ (1986) Age-dependent cyclosporin: pharmacokinetics in marrow transplant recipients. Clin Pharmacol Ther 40:438–443
Ptachcinski RJ, Burckart GJ, rosenthal JT, Venkataramanan R, Howrie DL, Taylor RJ, Avner ED, Ellis D, Hakala TR (1986) Cyclosporin pharmacokinetics in children following cadaveric renal transplantation. Transplant Proc 18:766–767
Morse GD, Holdsworth MT, Venuto RC, Gerbasi J, Walshe JJ (1988) Pharmacokinetics and clinical tolerance of intravenous and oral cyclosporine in the immediate postoperative period. Clin Pharmacol Ther 44:654–664
Lokiec F, Fischer A, cluckman E (1986) A safer approach to the clinical use of cyclosporine: the predose calculation. Transplant Proc 18:194–199
Clardy CW, Schroeder TJ, Myre SA, Wadhwa NK, Pesce AJ, First MR, McEnery PT, Balisteri WF, Harris RE, Melvin DB (1988) Clinical variability of cyclosporine pharmacokinetics in adult and pediatric patients after renal, cardiac, hepatic, and bone-marrow transplants. Clin Chem 34:2012–2015
Holmberg C, Jalanko H, Koskimies O, Leijala M, Salmela K, Eklund B, Wikström S, Ahonen J (1990) Renal transplantation in children with congenital nephrotic syndrome of the Finnish type. Transplant Proc 22:158–159
Wallemacq PE, Lesne M (1987) A new automated HPLC analysis of cyclosporin A and G in human serum. J Chromatogr 413:131–140
Gibaldi M (1984) Biopharmaceutics and clinical pharmacokinetics 3rd edn. Lea and Febiger, Philadelphia
Ptachcinski RJ, Venkataramanan R, Burckart GJ (1986) Clinical pharmacokinetics of cyclosporin. Clin Pharmacokinet 11:107–132
Reynolds KL, Grevel J, Gibbons SY, Welsh MS, Rutzky LP, Kahan BD (1988) cyclosporine pharmacokinetics in uremic patients: influence of different assay methods. Transplant Proc 20:462–465
Awni WM, Kasiske BL, Heim-Duthoy K, Rao KV (1989) Long-term cyclosporine pharmacokinetic changes in renal transplant recipients: effects of binding and metababolism. Clin Pharmacol Ther 45:41–48
Venkataramanan R, Habucky K, Burckart GJ, Ptachcinski RJ (1989) Clinical pharmacokinetics in organ transplant patients. Clin Pharmacokinet 16:134–161
Lindholm A, Henricsson S, Lind M, Dahlqvist R (1988) Intraindividual variability in the relative systemic availability of cyclosporin after oral dosing. Eur J Clin Pharmacol 34:461–464
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hoppu, K., Koskimies, O., Holmberg, C. et al. Pharmacokinetically determined cyclosporine dosage in young children. Pediatr Nephrol 5, 1–4 (1991). https://doi.org/10.1007/BF00852828
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00852828