Abstract
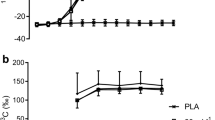
To determine if bypassing both intestinal absorption and hepatic glucose uptake by intravenous glucose infusion might increase the rate of muscle glucose oxidation above 1 g · min−1, ten endurance-trained subjects were studied during 125 min of cycling at 70% of peak oxygen uptake (VO2 peak). During exercise the subjects ingested either a 15 g · 100 ml−1 U-14C labelled glucose solution or H2O labelled with a U-14C glucose tracer for the determination of the rates of plasma glucose oxidation (Rox) and exogenous carbohydrate (CHO) oxidation from plasma14C glucose and14CO2 specific activities, and respiratory gas exchange. Simultaneously, 2-3H glucose was infused at a constant rate to measure glucose turnover, while unlabelled glucose (25% dextrose) was infused into those subjects not ingesting glucose to maintain plasma glucose concentration at 5 mmol · l−1. Despite similar plasma glucose concentrations [ingestion 5.3 (SEM 0.13) mmol · l−1; infusion 5.0 (0.09) mmol · l−1], compared to glucose infusion, CHO ingestion significantly increased plasma insulin concentrations [12.9 (1.0) vs 4.8 (0.5) mU · l−1;P<0.05], raised total Rox values [9.5 (1.2) vs 6.2 (0.7) mmol · 125 min−1 kg fat free mass−1 (FFM);P<0.05] and rates of CHO oxidation [37.2 (2.8)vs 24.1 (3.9) mmol · 125 min−1 kg FFM−1;P<0.05]. An increased reliance on CHO metabolism with CHO ingestion was associated with a decrease in fat oxidation. Whereas the contribution from fat oxidation to energy production increased to 51 (10)% with glucose infusion, it only reached 18 (4)% with glucose ingestion (P<0.05). Despite these differences in plasma insulin concentration and rates of fat oxidation, the rates of glucose oxidation by muscle were similar after 125 min of exercise for both trials [ingestion 93 (8); infusion 85 (5) μmol · min−1 kg FFM−1], suggesting that peak rates of muscle glucose oxidation were primarily dependent on blood glucose concentration which, in turn, regulated the hepatic appearance of ingested CHO.
Similar content being viewed by others
References
Ahlborg G, Felig P (1976) Influence of glucose ingestion on fuelhormone response during prolonged exercise. J Appl Physiol 41:683–688
Ahlborg G, Felig P (1982) Lactate and glucose exchange across the forearm, legs, and splanchnic bed during and after prolonged leg exercise. J Clin Invest 69:45–54
Allsop JR, Wolfe RR, Burke JF (1978) The reliability of rates of glucose appearance in vivo calculated from constant tracer infusions. Biochem J 172:407–416
Argoud GM, Schade DS, Eaton RP (1987) Underestimation of hepatic glucose production by radioactive and stable tracers. Am J Physiol 252: E606-E615
Balasse EO, Neef MA (1974) Operation of the “glucose-fatty acid cycle” during experimental elevation of plasma free fatty acid levels in man. Eur J Clin Invest 4:233–252
Bergman RN, Bier JR, Hourigan PM (1982) Intraportal glucose infusion matched to oral glucose absorption: lack of evidence for “gut factor” involvement in hepatic glucose storage. Diabetes 312:27–35
Bosch AN, Dennis SC, Noakes TD (1993) Influence of carbohydrate-loading on fuel substrate turnover and oxidation during prolonged exercise. J Appl Physiol 74:1921–1927
Broberg S, Sahlin K (1989) Adenine nucleotide degradation in human skeletal muscle during prolonged exercise. J Appl Physiol 67:116–122
Brooks GA (1986) Lactate production under fully aerobic conditions: the lactate shuttle during exercise and rest. Fed Proc 45:2924–2929
Coggan AR (1991) Plasma glucose metabolism during exercise in humans. Sports Med 11:102–124
Coggan AR, Coyle EF (1987) Reversal of fatigue during prolonged exercise by carbohydrate infusion or ingestion. J Appl Physiol 63:2388–2395
Coggan AR, Coyle EF (1991) Carbohydrate ingestion during prolonged exercise: effects on metabolism and performance. In: Holloszy JO (ed) Exercise and sports science reviews, vol 19. Williams and Wilkins, Baltimore, pp 1–40
Coggan AR, Kohrt WM, Spina RJ, Bier DM, Holloszy JO (1990) Endurance training decreases plasma glucose turnover and oxidation during moderate intensity exercise in men. J Appl Physiol 68:990–996
Coggan AR, Spina RJ, Kohrt WM, Bier D, Holloszy JO (1991) Plasma glucose kinetics in a well-trained cyclists fed glucose throughout exercise. Int J Sport Nutr 1:279–288
Consolazio CR, Johnson RE, Pecora LT (1963) Estimation of respiratory gases. In: Physiological measurements of metabolic functions in man. McGraw-Hill, New York, pp 72–87
Costill DL, Bennett A, Branham G, Eddy D (1973) Glucose ingestion at rest and during prolonged exercise. J Appl Physiol 34:764–769
Costill DL, Coyle E, Dalsky G, Evans W, Fink W, Hoopes D (1977) Effects of elevated plasma FFA and insulin on muscle glycogen usage during exercise. J Appl Physiol 43:695–699
Coyle EF, Coggan AR, Hemmert MK, Ivy JL (1986) Muscle glycogen utilization during prolonged strenuous execise when fed carbohydrate. J Appl Physiol 61:165–172
Coyle EF, Hamilton MT, Gonzalez Alonso J, Montain SJ, Ivy JL (1991) Carbohydrate metabolism during intense exercise when hyperglycemic. J Appl Physiol 70:834–840
DeFronzo R, Ferrannini E, Hendler R, Wahren J, Felig P (1978) Influence of hyperinsulinemia, hyperglycemia, and the route of glucose administration on splanchnic glucose exchange. Proc Natl Acad Sci 75:5173–5177
DeFronzo RA, Tobin JD, Andres R (1979) Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237: E214-E223
Durnin JVG, Wormersley J (1974) Body fat assessed from the total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr 32:77–97
Ferrannini EE, Barrett J, Bevilacqua S, DeFronzo RA (1983) Effect of fatty acids on glucose production and utilization in man. J Clin Invest 72:1737–1747
Finegood DT, Bergman RN, Vranic M (1987) Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes 36:914–924
Finegood DT, Bergman RN, Vranic M (1988) Modeling error and apparent isotope discrimination confound estimation of endogenous glucose production during euglycaemic glucose clamps. Diabetes 37:1025–1034
Gisolfi CV, Duchman SM (1992) Guidelines for optimal replacement beverages for different athletic events. Med Sci Sports Exerc 24:679–687
Goetz FC, Greenberg BZ (1961) A simple immunoassay for small amounts of insulin. J Clin Lab Med 58:819–822
Hargreaves M, Kiens B, Richter EA (1991) Effect of increased plasma free fatty acid concentrations on muscle metabolism in exercising men. J Appl Physiol 70:194–201
Hawley JA, Noakes TD (1992) Peak sustained power output predicts maximal oxygen uptake and performance time in trained cyclists. Eur J Appl Physiol 65:79–83
Hawley JA, Dennis SC, Noakes TD (1992a) Oxidation of carbohydrate ingested during prolonged endurance exercise. Sports Med 14:27–42
Hawley JA, Dennis SC, Nowitz A, Brouns F, Noakes TD (1992b) Exogenous carbohydrate oxidation from maltose and glucose ingested during prolonged exercise. Eur J Appl Physiol 64:523–527
Hawley JA, Bosch AN, Weltan SM, Dennis SC, Noakes TD (1994) Glucose kinetics during prolonged exercise in euglycaemic and hyperglycaemic subjects. Pflügers Arch 426:378–386
Jenkins AB, Chisholm DJ, James DE, Ho KY, Kraegen EW (1985) Exercise-induced hepatic glucose output is precisely sensitive to the rate of systemic glucose supply. Metabolism 34:431–436
Katz J, Dunn A (1967) Glucose-2 t as a tracer for glucose metabolism. Biochemistry 6:1–5
Katz A, Broberg S, Sahlin K, Wahren JH (1986) Leg glucose uptake during maximal dynamic exercise in humans. Am J Physiol 251: E65-E70
Radziuk J, Norwich KH, Vranic M (1978) Experimental validation of measurements of glucose turnover in non-steady state. Am J Physiol 234: E84-E93
Ravussin E, Bogardus C, Scheidegger K, Lagrange B, Horton ED, Horton ES (1986) Effect of elevated FFA on carbohydrate and lipid oxidation during prolonged exercise in humans. J Appl Physiol 60:893–900
Rennie MJ, Holloszy JO (1976) A sparing effect of increased free fatty acids on muscle glycogen content in exercising rat. Biochem J 156:647–655
Rennie MJ, Holloszy JO (1977) Inhibition of glucose uptake and glycogenolysis by availability of oleate in well-oxygenated perfused skeletal muscle. Biochem J 168:161–170
Romijn JA, Coyle EF, Sidossis L, Horowitz JF, Wolfe RR (1992) Effects of exercise on fat metabolism (abstract). Med Sci Sports Exerc [Suppl] 24: S72
Scherrer S, Haldimann B, Kupfer A, Reubi F, Bircher J (1978) Hepatic metabolism of aminopyrine in patients with chronic renal failure. Clin Sci Mol Med 54:133–140
Steele R (1959) Influence of glucose loading and of injected insulin on hepatic glucose output. Ann NY Acad Sci 82:420–430
Stein TP, Hoyt RW, O'Toole M, Leskiw MJ, Schluter MD, Wolfe RR, Hiller WBD (1989) Protein and energy metabolism during prolonged exercise in trained athletes. Int J Sports Med 10:311–316
Wahren J (1977) Glucose turnover during exercise in man. Ann NY Acad Sci 301:45–55
Walsh ML, Bannister EW (1988) Possible mechanism of the anaerobic threshold: a review. Sports Med 5:269–302
Wolfe BM, Klein S, Peters EJ, Schmidt BF, Wolfe RR (1988) Effect of elevated free fatty acids on glucose oxidation in normal humans. Metabolism 37:323–329
Wolfe RR, George S (1993) Stable isotopic traces as metabolic probes in exercise. In: Holloszy JO (ed) Exercise and sports science reviews, vol 21. Williams and Wilkins, Baltimore, pp 1–31
Wolfe RR, Nadel ER, Shaw JHF, Stephenson LA, Wolfe MH (1986) Role of changes in insulin and glucagon in glucose homeostasis in exercise. Metabolism 77:900–907
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hawley, J.A., Bosch, A.N., Weltan, S.M. et al. Effects of glucose ingestion or glucose infusion on fuel substrate kinetics during prolonged exercise. Europ. J. Appl. Physiol. 68, 381–389 (1994). https://doi.org/10.1007/BF00843733
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00843733