Summary
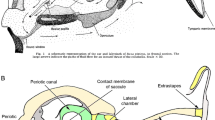
Intracellular dye-injection studies have revealed tonotopic organization of the bullfrog (Rana catesbeiana) amphibian papilla, an auditory organ lacking a basilar membrane or its equivalent. The best excitatory frequency (BEF) for auditory stimuli was identified in each of twenty-nine VIIIth-nerve afferent axons that subsequently were traced to their peripheral terminations at the sensory surface. Among those axons, the five with BEFs greater than 550 Hz all terminated in the caudalmost region of the papilla, the ten with the BEFs greater than 300 Hz and less than or equal to 550 Hz all terminated in the central region of the papilla, and the fourteen with BEFs equal to or less than 300 Hz all terminated in the rostralmost region of the papilla (Fig. 4). The tectorium is very much larger and presumably more massive under the low-frequency region of the papilla than it is under the high-frequency region (Fig. 1). Higher-frequency axons tended to innervate few (one to four) receptor cells, and low-frequency axons tended to innervate many (six or more). Higher-frequency axons often terminated in large claw-like structures that engulfed the basal portions of individual hair cells and in this way were morphologically similar to type I terminals in the inner ears of higher vertebrates.
Similar content being viewed by others
Abbreviations
- BEF :
-
best excitatory frequency
- HRP :
-
horseradish peroxidase
References
Békésy G von (1960) Experiments in hearing. Wever EG (ed). McGraw-Hill, New York
Bialek WS, Schweitzer AL (submitted) Thermal noise and the auditory hair cell. J Acoust Soc Am
Burlet HM de (1928) Über die Papilla neglecta. Anat Anz 66:199–209
Capranica RR, Moffat AJM (1974) Excitation, inhibition and ‘disinhibition’ in the inner ear of the toad (Bufo). J Acoust Soc Am 55:480
Capranica RR, Moffat AJM (1975) Selectivity of the peripheral auditory system of spadefoot toads (Scaphiopus couchi) for sounds of biological significance. J Comp Physiol 100:231–249
Dunn RF (1978) Nerve fibers of the eighth nerve and their distribution to the sensory nerves of the inner ear in the bullfrog. J Comp Neurol 182:621–636
Feng AS, Narins PM, Capranica RR (1975) Three populations of primary auditory fibers in the bullfrog (Rana catesbeiana): their peripheral origins and frequency sensitivities. J Comp Physiol 100:221–229
Flock Å, Flock B (1966) Ultrastructure of the amphibian papilla in the bullfrog. J Acoust Soc Am 40:1262
Frishkopf LS, Geisler CD (1966) Peripheral origin of auditory responses recorded from the eighth nerve of the bullfrog. J Acoust Soc Am 40:469–472
Frishkopf LS, Goldstein MH Jr (1963) Responses to acoustic stimuli from single units in the eighth nerve of the bullfrog. J Acoust Soc Am 35:1219–1228
Furukawa T (1978) Sites of termination on the saccular macula of auditory nerve fibers in the goldfish as determined by intracellular injection of Procion Yellow. J Comp Neurol 180:807–814
Geisler CD, Bergeijk WA van, Frishkopf LS (1964) The inner ear of the bullfrog. J Morphol 114:43–58
Harrison HS (1902) On the perilymphatic spaces of the amphibian ear. Int Mschr Anat Physiol 19:221–261
Landau LD, Lifshitz EM (1959) Fluid mechanics. Pergamon Press, Oxford, pp 88–98
Lewis ER (1976) Surface morphology of the bullfrog amphibian papilla. Brain Behav Evol 13:196–215
Lewis ER (1977) Structural correlates of function in the anuran amphibian papilla. In: Johari O, Becker RP (eds) Scanning electron microscopy/1977, vol II. IIT Res Inst. Chicago, pp 429–436
Liff H, Goldstein MH Jr, Frishkopf LS, Geisler CD (1968) Best inhibitory frequencies of complex units in the eighth nerve of the bullfrog. J Acoust Soc Am 44:635–636
Lombard RE (1980) The structure of the amphibian auditory periphery: a unique experiment in terrestrial hearing. In: Popper AN, Fay RR (eds) Comparative studies of hearing in vertebrates. Springer, New York Heidelberg Berlin, pp 121–138
Lombard RE, Bolt JR (1979) Evolution of the tetrapod ear: an analysis and reinterpretation. Biol J Linn Soc 11:19–76
Lombard RE, Straughan IR (1974) Functional aspects of anuran middle ear structures. J Exp Biol 61:71–93
Megela AL, Capranica RR (1979) Comparative studies of shortterm auditory adaptation and unit response patterns in eighth nerve of anurans. Soc Neurosci Abstr 5:27
Mulroy MJ (1974) Cochlear anatomy of the alligator lizard. Brain Behav Evol 10:69–87
Peake WT, Ling A Jr (1980) Basilar-membrane motion in the alligator lizard: its relation to tonotopic organization and frequency selectivity. J Acoust Soc Am 67:1736–1745
Retzius G (1881) Das Gehörorgan der Wirbeltiere, vol 1. Samson und Wallin, Stockholm
Stewart WW (1978) Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell 14:741–759
Turner RG, Nielsen DW (1980) Tuning curve properties and tonotopic organization of auditory nerve fibers in an iguanid and an anguid lizard. Assoc Res Otolaryngol Abstr 3:9
Turner RG, Muraski AA, Nielsen DW (1981) Cilium length: influence on neural tonotopic organization. Science 213:1519–1521
Weiss TF, Mulroy MJ, Turner RG, Pike CL (1976) Tuning of single fibers in the cochlear nerve of the alligator lizard: relation to receptor morphology. Brain Res 115:71–90
Weiss TF, Peake WT, Ling A Jr, Holton T (1978) Which structures determine frequency selectivity and tonotopic organization of vertebrate cochlear nerve fibers? Evidence from the alligator lizard. In: Naunton R, Fernandez C (eds) Evoked electrical activity in the auditory nervous system. Academic Press, New York, pp 81–112
Wersäll J (1956) Studies on the structures and innervation of the sensory epithelium of the cristae ampullares in the guinea pig. Acta Otolaryngol (Suppl) 126:1–85
Wersäll J, Flock Å, Lundquist PG (1965) Structural basis for directional sensitivity in cochlear and vestibular sensory receptors. Cold Spring Harbor Symp Quant Biol 30:115–145
Wever EG (1965) Structure and function of the lizard ear. J Aud Res 5:331–371
Wever EG (1973) The ear and hearing in the frog,Rana pipiens. J Morphol 141:461–478
Wever EG (1974) The evolution of vertebrate hearing. In: Keidel WD, Neff WD (eds) Handbook of sensory physiology, vol V/l. Springer, Berlin Heidelberg New York, pp 423–454
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lewis, E.R., Leverenz, E.L. & Koyama, H. The tonotopic organization of the bullfrog amphibian papilla, an auditory organ lacking a basilar membrane. J. Comp. Physiol. 145, 437–445 (1982). https://doi.org/10.1007/BF00612809
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00612809