Summary
Bovine cornea, sclera, iris, ciliary body, choroid, zonular fibers, lens capsule, lens nucleus, vitreous body, and retina were investigated for collagen content and type.
Cornea, sclera, iris, ciliary body, choroid, lens capsule, and vitreous body contain hydroxyproline, whereas in zonular fibers, lens nucleus, and retina no hydroxyproline was detectable.
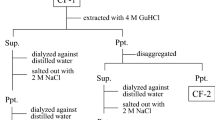
Preparative isolation of collagen was achieved by digestion of the different eye tissues with pepsin. The pepsin-solubilized collagen was separated by differential salt precipitation into different collagen types.
The polyacrylamide gel electrophoresis of the pepsin-solubilized collagens revealed type I collagen in cornea, sclera, iris, ciliary body, and choroid. As well as type I collagen, type III collagen was isolated from cornea, sclera, and uveal tissues. The identification of types I and III collagen was supported by the CNBr-derived peptides of these collagens. Lens capsule collagen consisted mainly of type IV collagen.
Zonular fibers contained no hydroxyproline but when examined by polyacrylamide gel electrophoresis, a band migrating in the α-position of collagen was observed. Polyacrylamide gel electrophoresis of both the pepsinsolubilized component and the CNBr-derived peptides of vitreous body protein showed no relation to any of the four common collagen types.
Zusammenfassung
Hornhaut, Lederhaut, Regenbogenhaut, Ziliarkörper, Aderhaut, Zonulafasern, Linsenkapsel, Linsenkern, Glaskörper und Netzhaut werden auf ihren Kollagengehalt untersucht. Auch soll festgestellt werden, welche Kollagentypen diese Gewebe aufbauen. Hornhaut, Lederhaut, Regenbogenhaut, Ziliarkörper, Aderhaut, Linsenkapsel und Glaskörper enthalten Hydroxyprolin. Hingegen ist in Zonulafasern, Linsenkern und Netzhaut kein Hydroxyprolin nachzuweisen. Aus den verschiedenen Augengeweben wurde mit Pepsin Kollagen gelöst, das durch Präzipitation mit NaCl-Lösungen verschiedener Molarität in verschiedene Kollagentypen getrennt werden kann.
Die Polyacrylamidgel-Elektrophorese des pepsin-gelösten Kollagens zeigte Type I Kollagen in Hornhaut, Lederhaut, Regenbogenhaut, Ziliarkörper und Aderhaut. Neben Typ I Kollagen wurde aus Hornhaut, Lederhaut und den Geweben der Uvea Typ III Kollagen isoliert. Typ I und Typ III Kollagen wurden durch die Peptide, die man mit CNBr-Spaltung erhalten kann, identifiziert. Die Linsenkapsel besteht hauptsächlich aus Typ IV Kollagen.
Zonulafasern enthalten kein Hydroxyprolin, zeigen aber in der Polyacrylamidgel-Elektrophorese eine Farblinie, die im α-Bereich von Kollagen wandert.
Die Polyacrylamidgel-Elektrophorese des pepsin-gelösten Kollagens des Glaskörpers sowie die CNBr-Spaltprodukte dieses Kollagens zeigten keine Identität mit einem der vier bisher isolierten Kollagentypen.
Similar content being viewed by others
References
Adelmann, B., Marquardt, H., Kühn, K.: Investigations on non-collagenous proteins in ratskin. Biochem. Z. 346, 282–296 (1966)
Bailey, A.J., Bazin, S., Sims, T.J., LeLous, M., Nicoletis, C., Delaunay, A.: Characterization of the collagen of human hypertrophic and normal scars. Biochim. Biophys. Acta 405, 412–421 (1975)
Barnes, M.J., Morton, L.F., Bennett, R.C., Bailey, A.J., Sims, T.J.: Presence of type III collagen in guinea-pig dermal scar. Biochem. J. 157, 263–266 (1976)
Bornstein, P., Piez, K.A.: Collagen: Structural studies based on the cleavage of methionyl bonds. Science 148, 1353–1355 (1965)
Chung, E., Miller, E.J.: Collagen polymorphism: Characterization of molecules with the chain composition [α1 (III)]3 in human tissues. Science 183, 1200–1201 (1974)
Drake, M.P., Davison, P.F., Bump, S., Schmitt, F.O.: Action of proteolytic enzymes on tropocollagen and insoluble collagen. Biochemistry 5, 301–312 (1966)
Freeman, I.L., Steven, F.S., Jackson, D.S.: Isolation and amino acid composition of bovine corneal polymeric collagens. Biochim. Biophys. Acta 154, 252–254 (1968)
Fujii, T., Kühn, K.: Isolation and characterization of pepsin-treated type III collagen from calf skin. Hoppe-Seyler's Z. Physiol. Chem. 356, 1793–1801 (1975)
Furthmayr, H., Timpl, R.: Characterization of collagen peptides by sodium dodecylsulfatepolyacrylamide electrophoresis. Anal. Biochem. 41, 510–516 (1971)
Gärtner, J.: The fine structure of the zonular fiber of the rat. Development and aging changes. Z. Anat.Entwickl.-Gesch. 130, 129–152 (1970)
Gloor, B.P.: Zur Entwicklung des Glaskörpers und der Zonula. VI. Autoradiographische Untersuchungen zur Entwicklung der Zonula der Maus mit 3H-markierten Aminosäuren und 3H-Glucose. Albrecht v. Graefes Arch. klin. exp. Ophthal. 189, 105–124 (1974)
Gross, J., Matoltsy, G., Cohen, C.: Vitrosin: A member of the collagen class. J. Biophys. Biochem. Cytol. 1, 215–220 (1955)
Gross, E., Witkop, B.: Selective cleavage of the methionyl peptide bonds in ribonuclease with cyanogen bromide. J. Amer. Chem. Soc. 83, 1510–1511 (1961)
Katzman, R.L., Kang, A.H., Dixit, S.N.: Isolation and characterization of bovine corneal collagen. Biochim. Biophys. Acta 336, 367–369 (1974)
Kefalides, N.A., Denduchis, B.: Structural components of epithelial and endothelial basement membranes. Biochemistry 8, 4613–4621 (1969)
Krause, A.C., Chan, E.: The chemical constitution of the conjunctiva, choroid and iris. Am. J. Physiol. 103, 270–274 (1933)
Matoltsy, A.G.: A study on the structural protein of the vitreous body (vitrosin). J. Gen. Physiol. 36, 29–37 (1952)
Miller, E.J.: Structural studies on cartilage collagen employing limited cleavage and solubilization with pepsin. Biochemistry 11, 4903–4909 (1972)
Olsen, B.R.: Electron microscope studies on collagen. IV. Structure of vitrosin fibrils and interaction properties of vitrosin molecules. J. Ultrastruct. Res. 13, 172–191 (1965)
Polatnick, J., LaTessa, A.J., Katzin, H.M.: Comparisons of collagen preparations from beef cornea and sclera. Biochim. Biophys. Acta 26, 365–369 (1957)
Propst, A., Hofmann, H.: Elektronenmikroskopische Untersuchungen der Zonulafasern mit besonderer Berücksichtigung der TrypsineinWirkung. Albrecht v. Graefes Arch. klin. exp. Ophthal. 162, 269–278 (1960)
Raviola, G.: The fine structure of the ciliary zonule and ciliary epithelium. With special regard to the organization and insertion of the zonular fibrils. Invest. Ophthalmol. 10, 851–869 (1971)
Rubin, A.L., Pfahl, D., Speakman, P.T., Davison, P.F., Schmitt, F.O.: Tropocollagen: Significance of protease induced alterations. Science 139, 37–39 (1963)
Schmut, O., Reich, M.E., Zirm, M.: Der Nachweis verschiedener Kollagentypen im Rinderauge. Albrecht v. Graefes Arch. klin. exp. Ophthal. 196, 71–77 (1975)
Schmut, O.: The identification of type III collagen in calf and bovine cornea and sclera. Exp. Eye. Res. 25, 505–509 (1977)
Smith, G.N., Jr., Linsenmayer, T.F., Newsome, D.A.: Synthesis of type II collagen in vitro by embryonic chick neural retina tissue. Proc. Natl. Acad. Sci. U.S.A. 73, 4420–4423 (1976)
Smits, G.: Quantitative interrelationships of the chief components of some connective tissues during foetal and post-natal development in cattle. Biochim. Biophys. Acta 25, 542–548 (1957)
Stegemann, H.: Mikrobestimmung von Hydroxyprolin mit Chloramin-T and p-Dimethylaminobenzal-dehyd. Hoppe-Seyler's Z. Physiol. Chem. 311, 41–45 (1958)
Swann, D.A., Constable, I.J., Harper, E.: Vitreous structure. III. Composition of bovine vitreous collagen. Invest. Ophthalmol. 11, 735–738 (1972)
Swann, D.A., Constable, I.J., Caulfield, J.B.: Vitreous structure. IV. Chemical composition of the insoluble residual protein fraction from the rabbit vitreous. Invest. Ophthalmol. 14, 613–616 (1975)
Swann, D.A., Caulfield, J.B., Broadhurst, J.B.: The altered fibrous form of vitreous collagen following solubilization with pepsin. Biochim. Biophys. Acta 427, 365–370 (1976)
Trelstad, R.L., Catanese, V.M., Rubin, D.F.: Collagen fractionation: Separation of native types I, II and III by differential precipitation. Anal. Biochem. 71, 114–118 (1976)
Uitto, J., Prockop, D.J.: Molecular defects in collagen and the definition of collagen disease. Mol. Pathol. 670–688 (1975)
Wollensak, J.: Zonula Zinnii. Histologische und chemische Untersuchungen, insbesondere über Zonolysis enzymatica und Syndroma Marfan. Fortschr. Augenheilk. 16, 240–335 (1965)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schmut, O. The organization of tissues of the eye by different collagen types. Albrecht von Graefes Arch. Klin. Ophthalmol. 207, 189–199 (1978). https://doi.org/10.1007/BF00411053
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00411053