Abstract
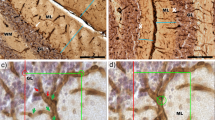
The present study systematically investigated the proportional evolution of Purkinje cell (PC) axonal swellings, also termed torpedoes, during aging of the two unrelated mouse strains B6CBA and C57BL/6J. Torpedoes were identified using monoclonal antibodies against the calcium-binding protein calbindin D-28k in mice ranging in age from 8 days postnatally up to 32 months. The relative density of PCs bearing torpedoes in animals up to 6 months of age was less than 0.1%. The number increases between 6–8 months and rises further in older mice almost linearly up to 13.7% affected PCs in the oldest animal (32 months) studied. In contrast, PC loss, as indicated by parvalbumin-immunoreactive empty baskets, is only at a very moderate level (less than 0.5%) in these strains. While the proximal axonic segments often show two and occasionally up to five swellings and frequently appear to be hypertrophied as a whole, the dendritic trees and neuronal somata of the affected PCs exhibit normal morphology. On rare occasions adaptive reactions indicated by “arciform axons” and enlarged varicosities of recurrent collaterals were observed. The results demonstrate that in addition to age-related PC loss of whatever degree, axonal disturbances of PCs, indicated by torpedoes, are present, leading most probably to a graded loss of cerebellar cortico-fugal projections.
Similar content being viewed by others
References
Andressen C, Blümcke I, Celio MR (1993) Calcium-binding proteins: selective markers of nerve cells. Cell Tissue Res 271: 181–208
Anthony DC, Boekelheide K, Giangaspero F, Allen JC Jr, Parks H, Priest JW, Webster D, Graham DG (1982) The neurofilament neuropathies: a unifying hypothesis. J Neuropathol Exp Neurol 41: 371
Aring CD (1938) Cerebellar syndrome in an adult with malformation of the cerebellum and brain stem (Arnold-Chiari deformity), with a note on the occurrence of “torpedoes” in the cerebellum. J Neurol Neurosurg Psychiatry 1: 100–109
Bakalian A, Corman B, Delhaye-Bouchaud N, Mariani J (1991) Quantitative analysis of the Purkinje cell population during ageing in the cerebellum of the wistar/louvain rat. Neurobiol Aging 12: 425–430
Batini C (1990) Cerebellar localization and colocalization of GABA and calicum binding protein-D28K. Arch Ital Biol 128: 127–149
Bäurle J, Grüsser-Cornehls U (1993) Age-related increase in axonal torpedoes in cerebellar Purkinje cells of two normal mouse strains. Soc Neurosci Abstr 19: 717.12
Bäurle J, Grüsser-Cornehls U (1994) Calbindin D-28k in the lateral vestibular nucleus of mutant mice as a tool to reveal Purkinje cell plasticity. Neurosci Lett 167: 85–88
Carpenter S, Karpati G, Andermann F, Told R (1974) Giant axonal neuropathy: a clinical and morphologically distinct neurological disease. Arch Neurol 31: 312–316
Celio MR, Baier W, Schärer L, De Viragh PA, Gerday C (1988) Monoclonal antibodies directed against the calcium binding protein parvalbumin. Cell Calcium 9: 81–86
Celio MR, Baier W, Schärer L, Gregersen HJ, De Viragh PA (1990) Monoclonal antibodies directed against the calcium binding protein Calbindin D-28k. Cell Calcium 11: 599–602
Delorenzi E (1931) Costanza numerica delle cellule del Purkinje in individui di varia eta. Boll Soc Ital Biol Sper 6: 80–82
Delorenzi E (1932) Costanza numerica delle cellule di Purkinje del cervelleto dell'uomo in individui di varia eta. Z Zellforsch Mikrosk Anat 14: 310–316
Druege H, Heinsen H, Heinsen YL (1986) Quantitative studies in aging Chbb: THOM (Wistar) rats. II. Neuron numbers in lobules I, Vlb+c and X. Bibl Anat 28: 121–137
Dumesnil-Bousez N, Sotelo C (1992) Early development of the Lurcher cerebellum: Purkinje cell alterations and impairment of synaptogenesis. J Neurocytol 21: 506–529
Ellis RS (1919) A preliminary quantitative study of the Purkinje cells in normal, subnormal and senescent human cerebella, with some notes on functional localization. J Comp Neurol 30: 229–252
Friedrich VL, Koniecki DL, Massa P (1980) Neuronal abnormalities in the cerebellum of quaking and shiverer mice. INSERM Symp 14: 141–146
Fujisawa K (1967) A unique type of axonal alteration (socalled axonal dystrophy) as seen in Goll's nucleus of 277 cases of controls. Acta Neuropathol (Berl) 8: 255–275
Gravel J, Leclerc N, Plioplys A, Hawkes B (1986) Focal axonal swellings in rat cerebellar Purkinje cells during normal development. Brain Res 363: 325–332
Guenet J-L, Sotelo C, Mariani J (1983) Hyperspiny Purkinje cell, a new neurological mutation in the mouse. J Hered 74: 105–108
Hall TC, Miller AKH, Corsellis JAN (1975) Variations in the human Purkinje cell population according to age and sex. Neuropathol Appl Neurobiol 1: 267–292
Hawkes R, Colonnier M, Leclerc N (1985) Monoclonal antibodies reveal sagittal banding in rodent cerebellar cortex. Brain Res 333: 359–365
Higashi Y, Murayama S, Pentchev PG, Suzuki K (1993) Cerebellar degeneration in the Niemann-Pick type C mouse. Acta Neuropathol 85: 175–184
Hirano A (1988) Color atlas of pathology of the nervous system, 2nd edn. Igaku-shoin, New York
Hsu SM, Ree HJ (1980) Self sandwich method: an improved immunoperoxidase technique for the detection of small amounts of antigens. Am J Clin Pathol 74: 32
Inukai J (1928) On the loss of Purkinje cells, with advancing age, from the cerebellar cortex of the albino rat. J Comp Neurol 45: 1–31
Jellinger K (1971) Neuroaxonal dystrophy in man: character and history. Acta Neuropathol (Berl) [Suppl] 5: 3–16
Kiefer R, Knoth R, Anagnostopoulos J, Volk B (1988) Cerebellar injury due to phenytoin. Identification and evolution of Purkinje cell axonal swellings in deep cerebellar nuclei of mice. Acta Neuropathol 77: 289–298
Kin (1934) Fukooha Ishi 35: 1167 (in Japanese), quoted by Takeya S (1970) Introduction to the Neuropathology. Igaku Shoin, Tokyo, p 170
Klatzo I (1968) Kuru Kuru. In: Minckler J (ed) Pathology of the nervous system, vol 1. McGraw-Hill, New York, pp 1161–1163
Lorke DE, Stan A, Lierse W (1989) Morphogenesis of the cerebellum of trisomy 19 mice. Biomed Res 10: 385–396
Mizushima S, Oyanagy S, Tsuyoshi I (1976) An ultrastructural observation of torpedoes in the human degenerative cerebellum. J Clin Electron Microsc 9: 672–673
Mullen RJ, Eicher EM, Sidman RL (1976) Purkinje cell degeneration, a new neurological mutation in the mouse. Proc Natl Acad Sci USA 73: 208–212
Nandy K (1981) Morphological changes in the cerebellar cortex of aging Macaca nemestrina. Neurobiol Aging 2: 61–64
Newborne PM, Hare WV (1962) Axonal dystrophy in clinically normal dogs. Am J Vet Res 23: 403–411
Ramón y Cajal S (1959) Degeneration and regeneration of the nervous system, vol II, Hafner, New York
Rogers J (1988) The neurobiology of cerebellar senescence. Ann NY Acad Sci 515: 251–268
Rogers J, Zornetzer SF, Bloom FE, Mervis RE (1984) Senescent microstructural changes in rat cerebellum. Brain Res 292: 23–32
Rosenfeld J, Friedrich VL Jr (1983) Axonal swellings in jimpy mice: does lack of myelin cause neuronal abnormalities? Neuroscience 10: 959–966
Sidman RL, Green MC, Appel SH (1965) Catalog of the neurological mutants of the mouse, Harvard University Press, Cambridge
Sotelo C (1990) Axonal abnormalities in cerebellar Purkinje cells of the “hyperspiny Purkinje cell” mutant mouse. J Neurocytol 19: 737–755
Sotelo C, Guenet JC (1983) Nodding a new mutant of the mouse with cerebellar abnormalities. Neurosci Lett [Suppl] 14: 353
Sotelo C, Triller A (1979) Fate of presynaptic afferents to Purkinje cells in the adult nervous mutant mouse: a model to study presynaptic stabilization. Brain Res 175: 11–36
Sotelo C, Wassef M (1991) Cerebellar development: afferent organization and Purkinje cell heterogeneity. Philos Trans R Soc Lond [Biol] 331: 307–313
Sturrock RR (1989) Changes in neuron number in the cerebellar cortex of the aging mouse. J Hirnforsch 30: 499–503
Sung JH (1964) Neuroaxonal dystrophy in mucoviscidosis. J Neuropath Exp Neurol 23: 567–583
Suzuki K, Zagoren JC (1975) Focal axonal swelling in cerebellum of Quaking mouse: light and electron microscopic studies. Brain Res 85: 38–43
Suzuki K, Andrews JM, Waltz JM, Terry RD (1969) Ultrastructural studies of multiple sclerosis. Lab Invest 20: 444–454
Takahashi N, Iwatsubo T, Nakano I, Machinami R (1992) Focal appearance of cerebellar torpedoes associated with discrete lesions in the cerebellar white matter. Acta Neuropathol 84: 153–156
Wassef M, Simons J, Tappaz ML, Sotelo C (1986) Non-Purkinje cell GABAergic innervation of the deep cerebellar nuclei: a quantitative immunocytochemical study in C57BL and in Purkinje cell degeneration mutant mice. Brain Res 399: 125–135
Woodruff-Pak DS, Sheffield JB (1987) Age differences in Purkinje cells and rate of classical conditioning in young and older rabbits. Soc Neurosci Abstr 13: 124.9
Zilla P, Celio MR, Fasol R, Zenker W (1985) Ectopic parvalbumin-positive cells in the cerebellum of the adult mutant mouse “nervous”. Acta Anat (Basel) 124: 181–187
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bäurle, J., Grüsser-Cornehls, U. Axonal torpedoes in cerebellar Purkinje cells of two normal mouse strains during aging. Acta Neuropathol 88, 237–245 (1994). https://doi.org/10.1007/BF00293399
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00293399