Abstract
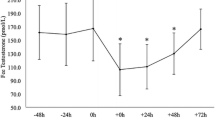
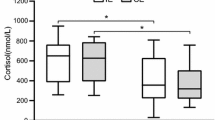
An increase in the amounts of circulating plasma cortisol or a decrease in testosterone can result in whole-body insulin resistance. The purpose of this study was to determine if the increase in cortisol and/or decrease in testosterone concentrations commonly evident with intense endurance training is associated with insulin resistance. Male (n = 9) and female (n = 10) swimmers were examined during the off-season, after 9 weeks (9 WKS) of training averaging 5,500 m·day−1 and after an additional 9 weeks (18 WKS) of training averaging 8,300 m·day−1. Resting plasma cortisol concentration was (P <− 0.05) higher in the women compared to the men at 9 WKS; values were not significantly different between genders at 18 WKS. Plasma testosterone concentration decreased significantly (P <− 0.05) in the men at 9 and 18 WKS, but did not change in the women. Whole-body insulin action, as determined by insulin and glucose responses during a 120 min, 75-g oral glucose tolerance test, did not change with training in either the men or women. These data indicated that plasma testosterone concentration can decrease in male swimmers during intense endurance training; this alteration does not affect whole-body insulin action. There would also appear to be a gender-specific response of plasma cortisol to endurance training, which does not influence insulin action.
Similar content being viewed by others
References
American Diabetes Association (1989) The physicians guide to type II diabetes (NIDDM): Diagnosis and treatment. American Diabetes Association, Alexandria, Va, pp 3–12
Anderson DC (1974) Sex-hormone-binding-globulin. Clin Endocrinol 3:69–96
Barron JL, Noakes TD, Levy W, Smith S, Millar RP (1985) Hypothalamic dysfunction in overtrained athletes. J Clin Endocrinol Metab 60:803–806
Bjorntorp P (1989) Hyperandrogenicity and insulin resistance as predictors for non-insulin-dependent diabetes mellitus in women. In: Alberti KGMM, Mazze R (eds) Frontiers of diabetes research: current trends in non-insulin-dependent diabetes mellitus. Elsevier Science, Amsterdam pp 61–70
Bouchard C, Tremblay A, Leblanc C, Lortie G, Savard R, Theriault G (1983) A method to assess energy expenditure in children and adults. Am J Clin Nutr 37:461–467
Cederholm J, Wibell L (1985) Evaluation of insulin release and relative peripheral resistance with use of the oral glucose tolerance test: a study in subjects with normoglycaemia, glucose intolerance and non-insulin-dependent diabetes mellitus. Scand J Clin Lab Invest 45:741–751
Costill PL, Thomas R, Rebergs RA, Pascoe P, Lamber TC, Barr S, Fink WJ (1991) Adaptations to swimming training: influence of training volume. Med Sci Sports Exercise 23:371–377
Flynn MG, Pizza FX, Boone JB, Andres FF, Michaud TA, Rodriguez-Zayas JR (1994) Indices of training stress during competitive running and swimming seasons. Int J Sports Med 15:21–26
Guillaume-Gentil C, Assimacopoulos-Jeannet F, Jeanrenaud B (1993) Involvement of non-esterified fatty acid oxidation in glucocorticoid-induced peripheral insulin resistance in vivo in rats. Diabetolgia 36:899–906
Haffner SM, Karhapaa P, Mykkanen L, Laakso M (1994) Insulin resistance, body fat distribution, and sex hormones in men. Diabetes 43:212–219
Hoffer LJ, Beitins IZ, Kyung NH, Bistrain BR (1986) Effects of severe dietary restriction on male reproductive hormones. J Clin Endocrinol Metab 62:288–292
Holmang A, Bjorntorp P (1992a) The effects of cortisol on insulin sensitivity in muscle. Acta Physiol Scand 144:425–431
Holmang A, Bjorntorp P (1992b) The effects of testosterone on insulin sensitivity in male rats. Acta Physiol Scand 146:505–510
Houmard JA, McCulley C, Shinebarger MH, Bruno NJ (1994) Effects of exercise training on plasma androgens in men. Horm Metab Res 26:297–300
Ivy JL (1987) The insulin-like effect of muscle contraction. In: Pandolf KB (ed) Exercise and sport sciences reviews. Macmillan, New York, pp 29–51
Monpetit RR, Leger LA, Lavoie JM, Carzorla GA (1981) \(\dot V{\text{O}}_{{\text{2peak}}} \) during free swimming using the backward extrapolation of the O2 recovery curve. Eur J Appl Physiol 47:385–391
Opstad PK (1992) Androgenic hormones during prolonged physical stress, sleep, and energy deficiency. J Clin Endocrinol Metab 74:1176–1183
Phillips GB (1993) Relationship between serum sex hormones and the glucose-insulin-lipid defect in men with obesity. Metabolism 42:116–120
Rooney DP, Neely RDG, Cullen C, Ennis CN, Sheridan B, Atkinson AB, Trimble ER, Bell PM (1993) The effect of cortisol on glucose/glucose-6-phosphate cycle activity and insulin action. J Clin Endocrinol Metab 77:1180–1183
Seidell JC, Bjorntorp P, Sjostrom L, Kvist H, Sannerstedt R (1990) Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels but negatively with testosterone levels. Metabolism 39:897–901
Stanik S, Dornfeld LP, Maxwell MH, Viosca SP, Korenman SG (1981) The effect of weight loss on reproductive hormones in obese men. J Clin Endocrinol Metab 53:828–832
Strauss RH, Lanese RR, Malarkey WB (1993) Decreased testosterone and libido with severe weight loss. Phys Sportsmed 12:64–71
Tegelman R, Johansson C, Hemmingsson P, Eklof R, Carlstrom K, Pousette A (1989) Endogenous anabolic and catabolic steroid hormones in male and female athletes during off season. Int J Sports Med 10:103–106
Tsai L, Johansson C, Pousette A, Tegelman R, Carlstrom K, Hemmingsson P (1991) Cortisol and androgen concentrations in female and male elite endurance athletes in relation to physical activity. Eur J Appl Physiol 63:308–311
Vervoorn C, Vermulst LJM, Boelens-Quist AM, Koppeschaar HPF, Erick WBM, Thijssen JHH, de Vries WR (1991) Seasonal changes in performance and free testosterone: cortisol ratio of elite female rowers. Eur J Appl Physiol 64:14–21
Villanueva AL, Schlosser C, Hopper B, Liu JH, Hoffman DI, Rebar RW (1986) Increased cortisol production in women runners. J Clin Endocrinol Metab 63:133–136
Wheeler GD, Singh M, Pierce WD, Epling WF, Cumming DC (1991) Endurance training decreases serum testosterone level in men without change in luteinizing hormone pulsatile release. J Clin Endocrinol Metabol 72:422–425
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tyndall, G.L., Kobe, R.W. & Houmard, J.A. Cortisol, testosterone, and insulin action during intense swimming training in humans. Europ. J. Appl. Physiol. 73, 61–65 (1996). https://doi.org/10.1007/BF00262810
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00262810