Summary
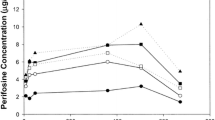
A total of 14 patients, 7 male and 7 female, received in all 21 evaluable courses of cyclophosphamide administered by 5-day continuous infusion. Cyclophosphamide doses were escalated from 300 to 400 mg/m2 per day for 5 days and repeated every 21–28 days. The patient population had a median age of 55 years (range 38–76) and a median Karnofsky performance status of 80 (range 60–100). Only 1 patient had not received prior therapy; 5 patients had received only prior chemotherapy, 1 had received only prior radiotherapy, and 7 had received both. Tumor types were gastric (1), lung (2), colon (4), urethral adenocarcinoma (1), cervical (2), chondrosarcoma (1), melanoma (1), uterine leiomyosarcoma (1), and pancreatic (1). The dose-limiting toxicity was granulocytopenia, with median WBC nadir of 1700/μl (range 100–4800) in 8 heavily pretreated patients treated at 350 mg/m2 per day for 5 days. One patient without heavy prior treatment received two courses at 400 mg/m2 and had WBC nadirs of 800/μl and 600μl. WBC nadirs occurred between days 9 and 21 (median 14). Drug-induced thrombocytopenia occurred in only one patient (350 mg/m2 per day, nadir 85000/μl). Neither hyponatremia nor symptomatic hypoosmolality was observed. Radiation-induced hemorrhagic cystitis may have been worsened in one patient. Nausea and vomiting were mild. Objective remissions were not observed. The maximum tolerated dose for previously treated patients is 350 mg/m2 per day for 5 days. This dose approximates the doses of cyclophosphamide commonly used with bolus administration. Plasma steady-state concentrations (Css) of cyclophosphamide, measured by gas liquid chromatography, were 2.09–6.79 μg/ml. Steady state was achieved in 14.5±5.9 h (mean ±SD). After the infusion, cyclophosphamide disappeared from plasma monoexponentially, with a t1/2 of 5.3±3.6 h. The area under the curve of plasma cyclophosphamide concentrations versus time (AUC) was 543±150 μg/ml h and reflected a cyclophosphamide total-body clearance (CLTB) of 103±31.6 ml/min. Plasma alkylating activity, assessed by p-nitrobenzyl-pyridine, remained steady at 1.6–4.3 μg/ml nor-nitrogen mustard equivalents. Urinary excretion of cyclophosphamide and alkylating activity accounted for 9.3%±7.6% and 15.1%±2.0% of the administered daily dose, respectively. The t1/2 and AUC of cyclophosphamide associated with the 5-day continuous infusion schedule are similar to those reported after administration of cyclophosphamide 1500 mg/m2 as an i.v. bolus. The AUC of alkylating activity associated with the 5-day continuous infusion of cyclophosphamide is about three times greater than the AUC of alkylating activity calculated after a 1500-mg/m2 bolus dose of cyclophosphamide. Daily urinary excretions of cyclophosphamide and alkylating activity associated with the 5-day continuous infusion schedule are similar to those reported after bolus doses of cyclophosphamide.
Similar content being viewed by others
References
Aisner J, Van Echo D, Whitacre M, Wiernik P (1982) A phase I trial of continuous infusion VP16-213 (etoposide). Cancer Chemother Pharmacol 7: 157
Begley CM Jr, Bostick FW, DeVita VT Jr (1973) Clinical pharmacology of cyclophosphamide. Cancer Res 33: 226
Bedikian AJ, Bodey GP (1983) Phase I study of cyclophosphamide (NSC 28271) by 72-hour continuous intravenous infusion. Am J Clin Oncol 6: 365
Bennet JM, Reich SD (1979) Bleomycin. Ann Intern Med 90: 945
Bodey GP, Yap HY, Yap BS, Valdivieso M (1980) Continuous infusion vindesine in solid tumors. Cancer Treat Rev 7 (Suppl): 39
Bruce WR, Meeker BE, Valeriote FA (1966) Comparison of the sensitivity of normal hematopoietic and transplanted lymphoma colony-forming cells to chemotherapeutic agents administered in vivo. J Natl Cancer Inst 37: 233
Cassileth PA, Katz ME (1977) Chemotherapy for adult acute non-lymphocytic leukemia with daunorubicin and cytosine arabinoside. Cancer Treat Rep 61: 1441
Cohen JL, Lao JY, Jusko WJ (1971) Pharmacokinetics of cyclophosphamide in man. Br J Pharmacol 43: 677
D'Incalci M, Bolis G, Facchinetti T, Mangioni C, Morascol L, Morazzoni P, Salmona M (1979) Decreased half-life of cyclophosphamide in patients under continual treatment. Eur J Cancer 19: 7
Friedman OM, Boger E (1961) Colorimetric estimation of nitrogen mustards in aqueous media. Anal Chem 33: 906
Fuks JZ, Egorin MJ, Aisner J, Ostrow SS, Klein ME, Bachur NR, Colvin M, Wiernik PH (1981) Cyclophosphamide of dimethylsulfoxide in the treatment of squamous carcinoma of the lung. Cancer Chemother Pharmacol 6: 117
Grochow LB, Colvin OM (1979) Clinical pharmacokinetics of i.v. cyclophosphamide. Clin Pharmacokinet 4: 380
Jardine I, Fenselau C, ppler M, Kan MN, Brundrett RB, Colvin M (1978) Quantitation by gas chromatography-chemical ionization mass spectrometry of cyclophosphamide, mustard, and nornitrogen mustard in the plasma and urine of patients receiving cyclophosphamide therapy. Cancer Res 38: 408
Juma FD, Rogers KJ, Trounce JR, Brodbook JD (1978) Pharmacokinetics of intravenous cyclophosphamide in man estimated by gas-liquid chromatography. Cancer Chemother Pharmacol 1: 229
Juma FD, Rogers JH, Trounce JR (1979) Pharmacokinetics of cyclophosphamide and alkylating activity in man after intravenous and oral administration. Br J Clin Pharmacol 8: 209
Klein HO, wickramanayake PD, Christian E, Coerper C (1984) Therapeutic effects of single-push or fractionated injections or continuous infusion of oxazaphosphorines (cyclophosphamide, ifosfamide, Asta 27557). Cancer 54: 1193
Knott GD, (1979) MLAB, A mathematical model tool. Comput Prog Biomed 10: 271
Lee EJ, Van Echo DA, Egorin MJ, Nayar MSB, Shulman P, Schiffer CA (1986) Diaziquone given as a continuous infusion is an active agent for relapsed adult acute nonlymphocytic leukemia. Blood 67: 182
Legha SS, Benjamin RS, Mackay B, Ewer M, Wallace S, Valdivieso M, Rasmussen SL, Blumenschein GR, Freireich EJ (1982) Reduction of doxorubicin cardiotoxicity by prolonged continuous intravenous infusion. Ann Intern Med 96: 133
Mouridsen HT, Faber O, Skovsted L (1974) The biotransformation of cyclophosphamide in man: Analysis of the variation in normal subjects. Acta Pharmacol Toxicol (Copenh) 35: 98
Salidoro A, Otero J, Vallejos C, Casanova L, Salas F, Pasco T, Quinoz L, Orlandini O, Marcial J (1981) Intermittent continuous i.v. infusion of high-dose cyclophosphamide for remission induction in acuty lymphocytic leukemia. Cancer Treat Rep 65: 213
Seifert P, Baker L, Reed M, Vaitkevicius VK (1975) Comparison of continuously infused 5-fluorouracil with bolus injection in the treatment of patients with colorectal adenocarcinoma. Cancer 36: 123
Stuart-Harris R, Harper PG, Kaye SB, Wiltshaw E (1983) High-dose ifosfamide by infusion with mesna in advanced soft tissue sarcoma. Cancer Treat Rev 10: 163
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tchekmedyian, N.S., Egorin, M.J., Cohen, B.E. et al. Phase I clinical and pharmacokinetic study of cyclophosphamide administered by five-day continuous intravenous infusion. Cancer Chemother. Pharmacol. 18, 33–38 (1986). https://doi.org/10.1007/BF00253060
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00253060